
(a)
Interpretation:
The IUPAC name for the ester formed when acetic acid and ethanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of
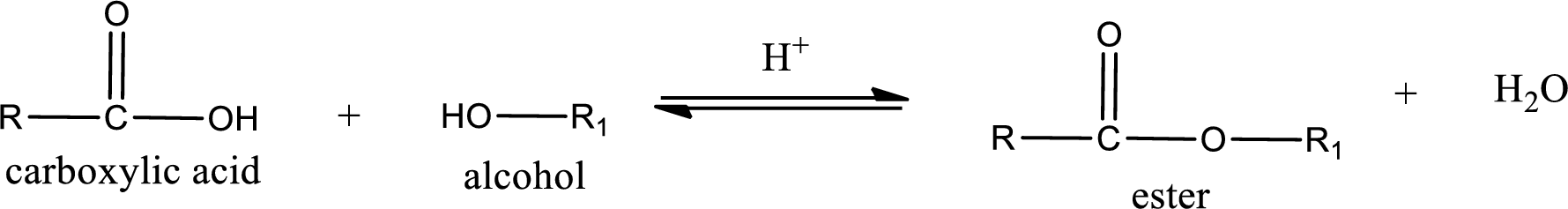
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
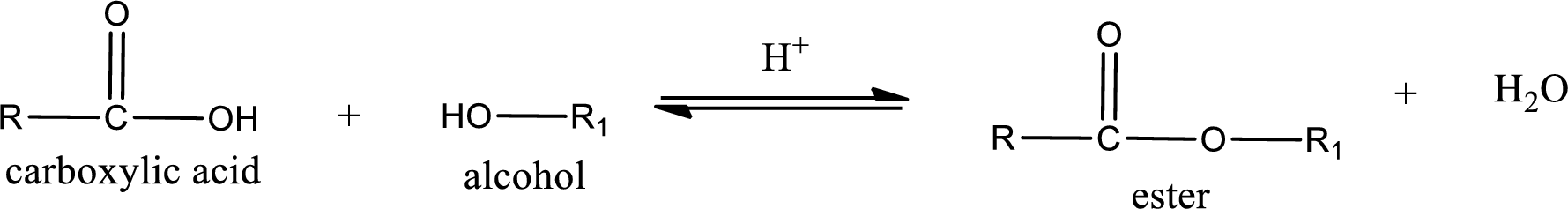
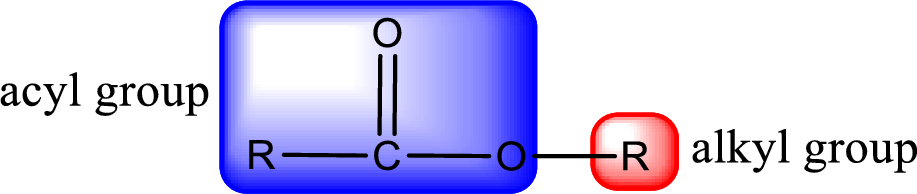
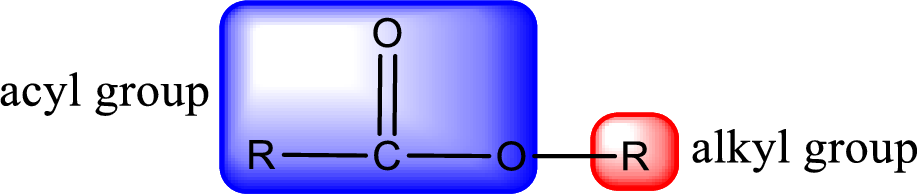
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
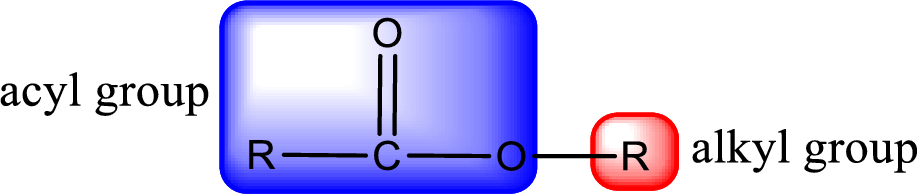
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(a)
Answer to Problem 16.97EP
IUPAC name of the ester formed is ethyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
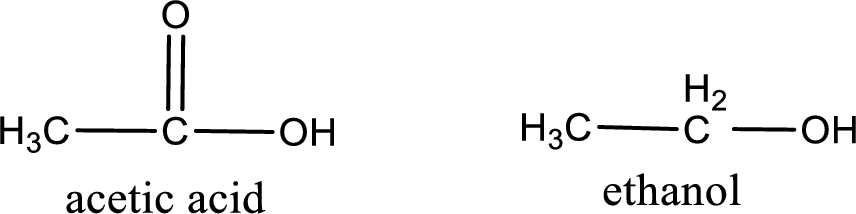
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
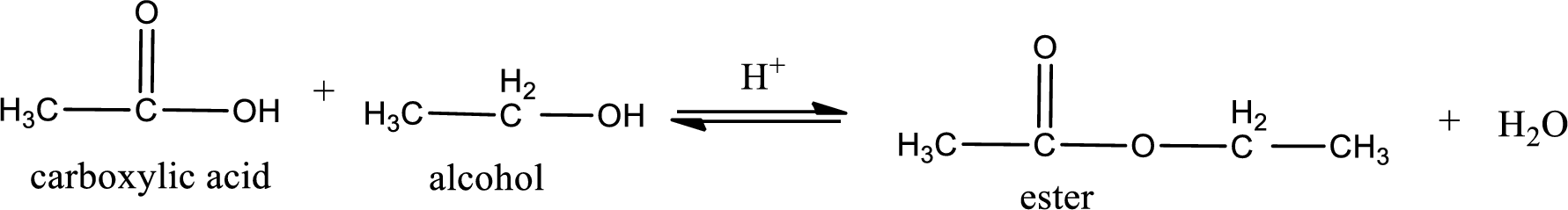
The structure of ester is,
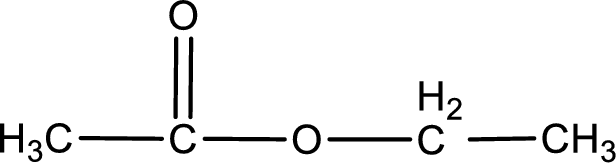
The alkyl and acyl group is identified as shown below,
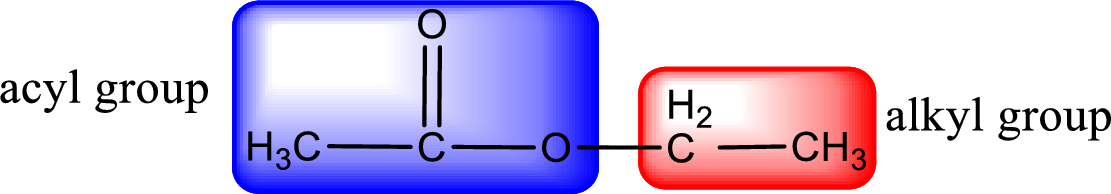
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is ethyl ethanoate.
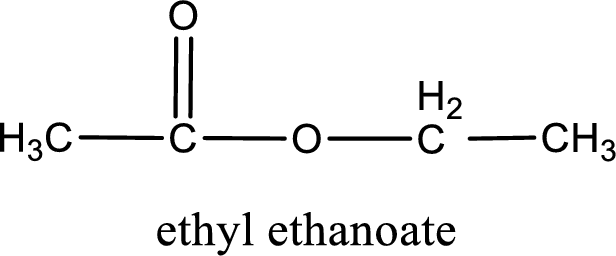
IUPAC name of the formed ester is assigned.
(b)
Interpretation:
The IUPAC name for the ester formed when ethanoic acid and methanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
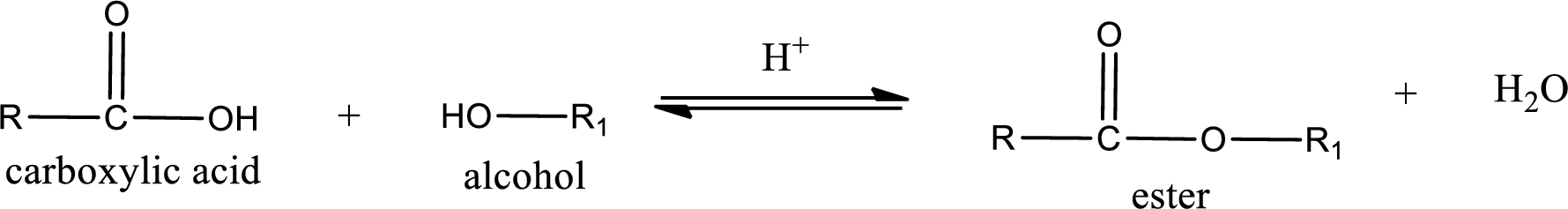
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(b)
Answer to Problem 16.97EP
IUPAC name of the ester formed is methyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
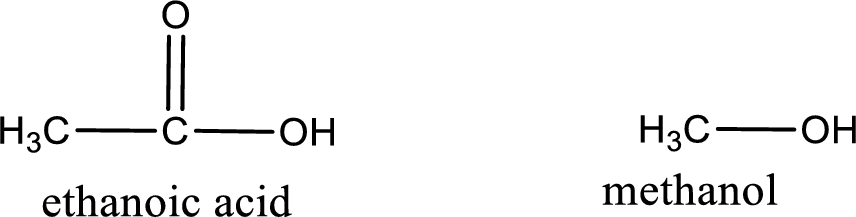
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
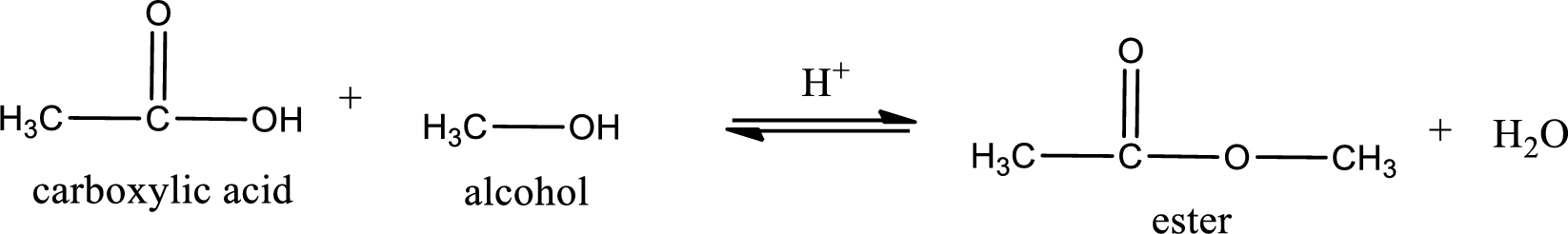
The structure of ester is,
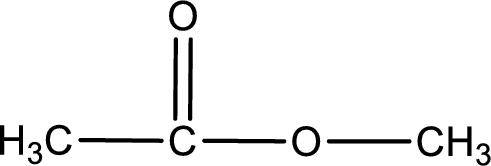
The alkyl and acyl group is identified as shown below,
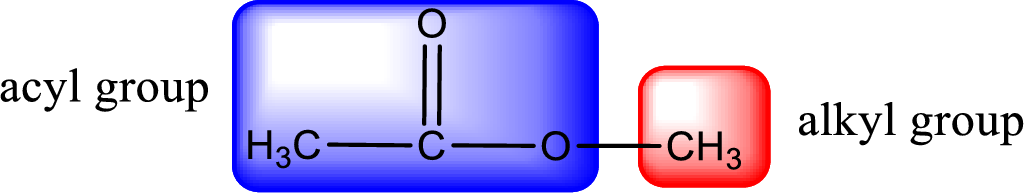
Alkyl group contains only one carbon atom. Hence alkyl group is named as methyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is methyl ethanoate.
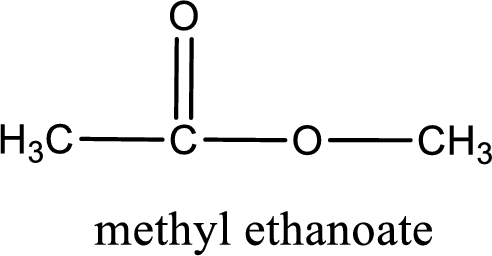
IUPAC name of the formed ester is assigned.
(c)
Interpretation:
The IUPAC name for the ester formed when butyric acid and ethyl alcohol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
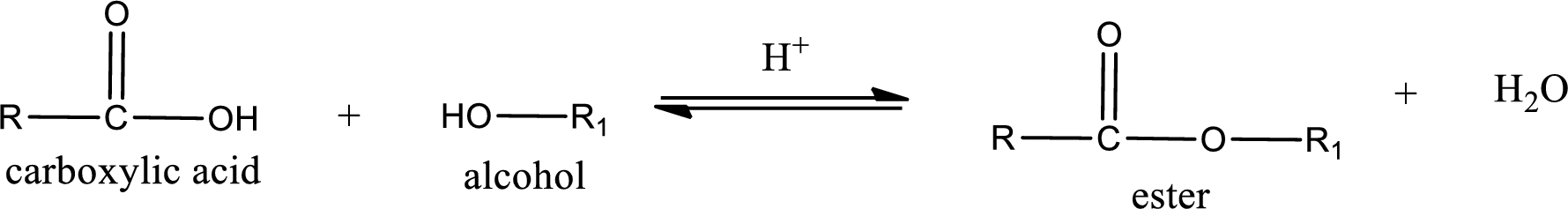
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
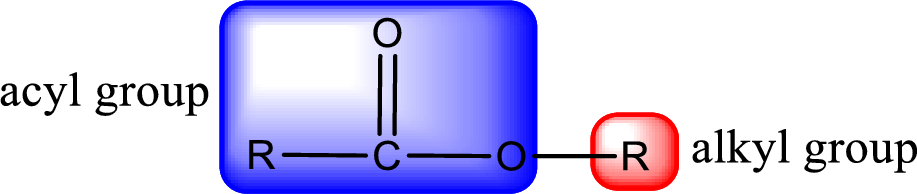
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(c)
Answer to Problem 16.97EP
IUPAC name of the ester formed is ethyl butanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
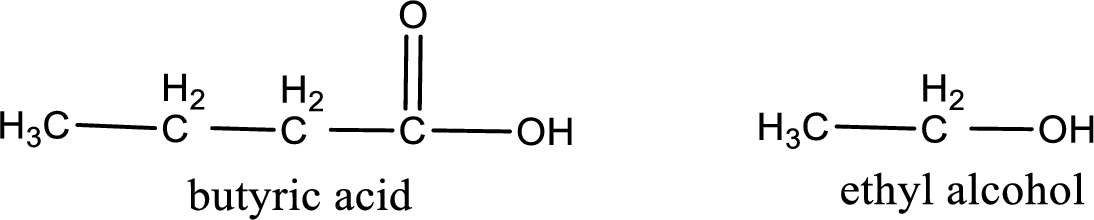
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
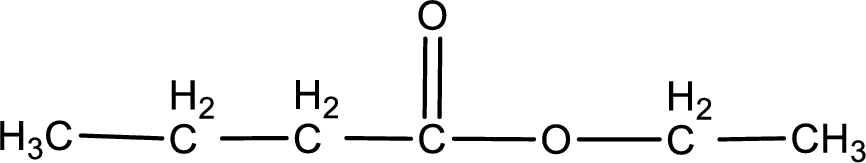
The alkyl and acyl group is identified as shown below,
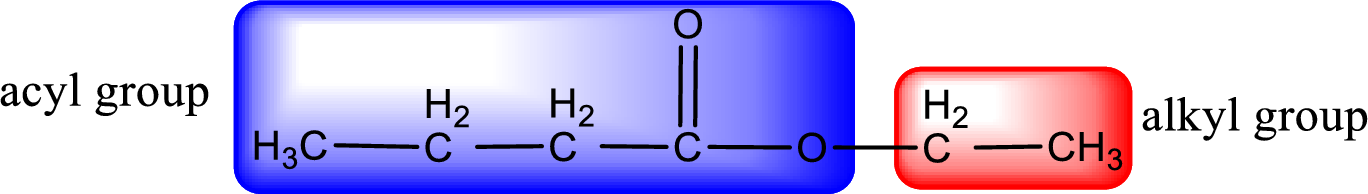
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains four carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is butanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as butanoate. Therefore IUPAC name of the given ester is ethyl butanoate.
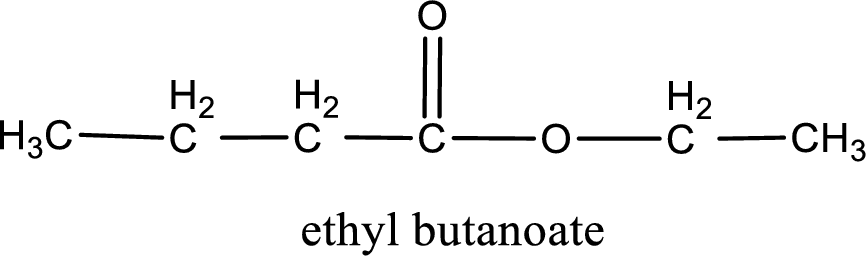
IUPAC name of the formed ester is assigned.
(d)
Interpretation:
The IUPAC name for the ester formed when 2-butanol and caproic acid react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(d)
Answer to Problem 16.97EP
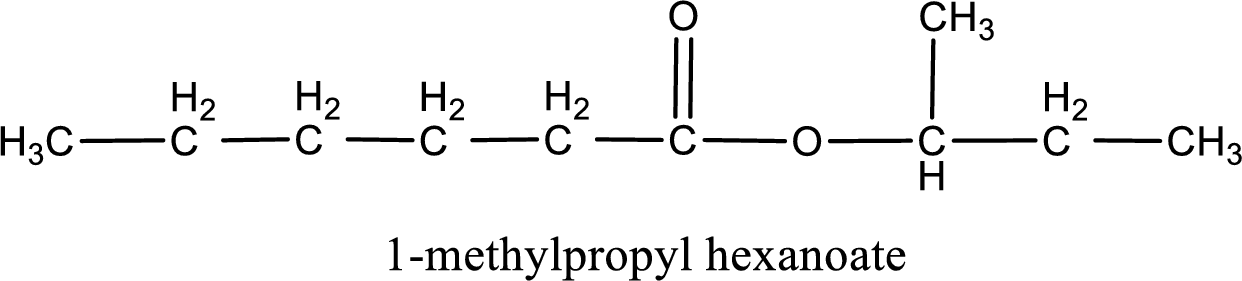
IUPAC name of the ester formed is 1-methylpropyl hexanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
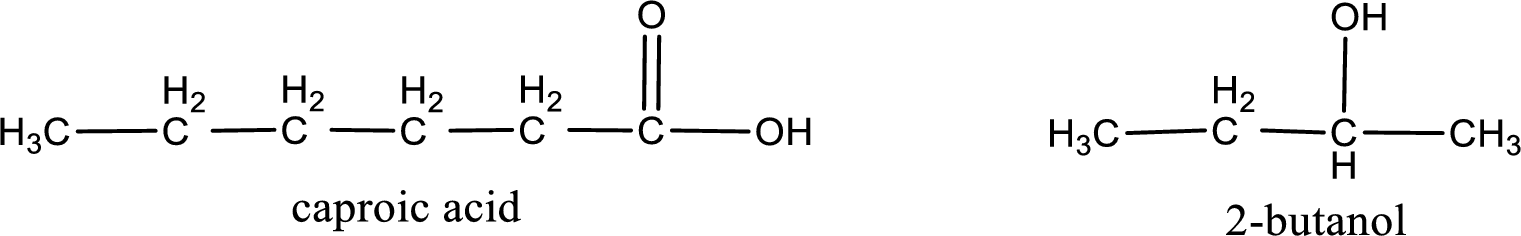
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
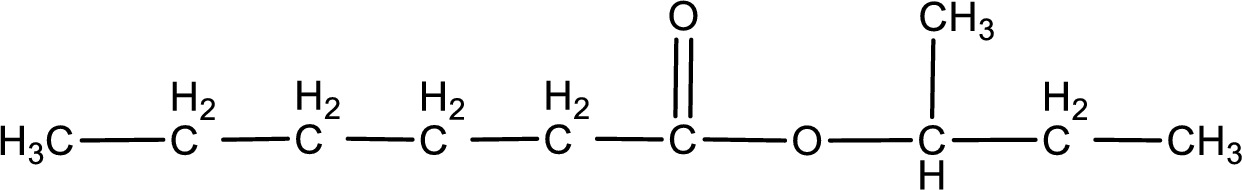
The alkyl and acyl group is identified as shown below,
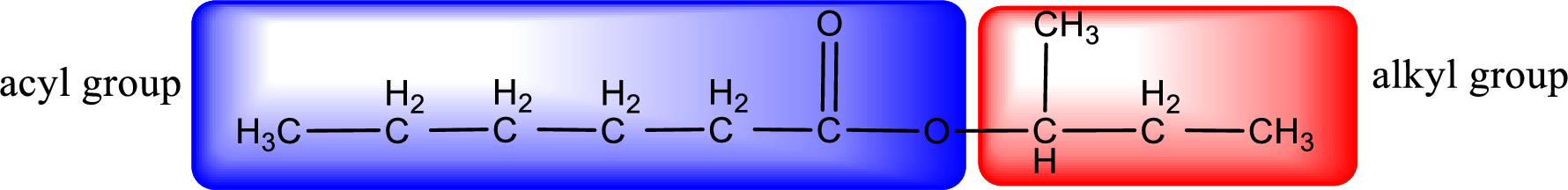
Alkyl group contains a total of four carbon atoms. In that alkyl group (propyl) a methyl group is substituted in the first carbon atom. Hence alkyl group is named as 1-methylpropyl. The acyl group contains six carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is hexanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as hexanoate. Therefore IUPAC name of the given ester is 1-methylpropyl hexanoate.
IUPAC name of the formed ester is assigned.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





