
Concept explainers
(a)
Interpretation:
Structural formula for methyl thioethanoate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
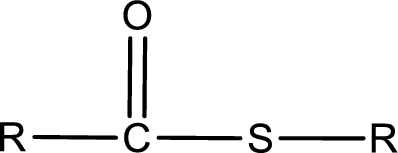
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(b)
Interpretation:
Structural formula for methyl thioacetate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
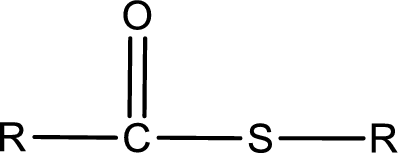
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(c)
Interpretation:
Structural formula for ethyl thioformate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
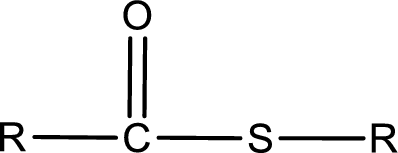
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
(d)
Interpretation:
Structural formula for ethyl thiomethanoate has to be drawn.
Concept Introduction:
General structure of thioester can be represented as shown below,
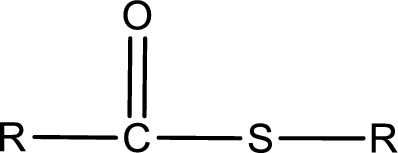
From an IUPAC name, the structure of the thioester can be derived. IUPAC name of thioester consists of two parts. In an IUPAC name of thioester, the first part of the name is the alkyl part and the second part is the acid part. Alkyl group have come from the thiol and acyl part from carboxylic acid.
The same rule applies for deriving a structure from common name. The only difference is the acyl part name. The acyl part is named using the common name of thiocarboxylic acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, And Biological Chemistry, Hybrid (with Owlv2 Quick Prep For General Chemistry Printed Access Card)
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





