
Concept explainers
Practice Problem 16.14
Dihydropyran reacts readily with an alcohol in the presence of a trace of anhydrous HCL or
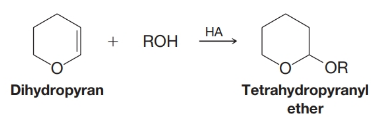
(a) Write a plausible mechanism for this reaction.
(b) Tetrahydropyranyl ethers are stable in aqueous base but hydrolyze rapidly in aqueous acid to yield the original alcohol and another compound. Explain. (What is the other compound?)
(c) The tetrahydropyranyl group can be used as a protecting group for alcohols and phenols. Show how you might use it in a synthesis of 5-methyl-1,5-hexanediol starting with 4-chloro-1-butanol.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Fundamentals Of Thermodynamics
Living By Chemistry: First Edition Textbook
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Applications and Investigations in Earth Science (9th Edition)
Microbiology: An Introduction
- 1. Sodium azide (NaN3) is the primary chemical substance used in automobile air bags. Upon impact, the decomposition of sodium azide is initiated to produce sodium metal and nitrogen gas which then inflates the bag. How many liters of nitrogen gas are produced at 1.15 atm and 30.0°C when 145.0 grams of sodium azide decomposes? 2. Calcium carbonate (such as that in limestone) reacts with aqueous hydrochloric acid to produce carbon dioxide, aqueous calcium chloride and water. How many liters of carbon dioxide are produced at 20°C and 745 torr when 3.583 grams of calcium carbonate is dissolved in solution containing 1.550 grams of hydrochloric acid?arrow_forwardShow all work (where appropriate) for full credit. 1. Describe (steps, equipment and quantities) how to accurately prepare 250.0 mL of a 0.0075 M solution of NaCl (aq) from a 500 mL, 0.0500 M stock solution. 2. Describe (steps, equipment and quantities) how to accurately prepare 250.0 mL of a 0.0075 M solution of NaCl (aq) from 100 g of solid NaCl.arrow_forward5. An unlabeled gas cylinder was recently found in the laboratory. A sample of the gas was removed and analyzed. A 500.0 mL sample of the gas at 15°C and a pressure of 736 mmHg was found to weigh 2.688 g. Determine the molar mass of the gas. What element is the gas?arrow_forward
- 4. Nitrogen gas is commonly sold in 49.0 L steal cylinders at a pressure of 150 atm. (a) How many moles of nitrogen are in the container if the temperature of the cylinder is 21°C. (b) How many moles of nitrogen will there be if the container above is heated to 100°C? (careful here) (c) What is the mass of nitrogen gas in the cylinder in part (a)? (d) What volume would the nitrogen occupy at 21°C, if the pressure was reduced to 1.02 atm? (e) What would be the pressure of the nitrogen gas in the cylinder when the temperature is raised to 39°C?arrow_forward6. A 0.4550 g sample of an unknown organic compound with the empirical formula CH2O was placed into a 150.0 ml vessel and was vaporized into a gas. At 175.0°C, the pressure of the vaporized compound was measured at 941.1 torr. (a) Determine the molar mass of the compound (b) Determine the molecular formula of the compound.arrow_forwardDon't used Ai solutionarrow_forward
- 3. A particular reaction calls for 2.40 g of chloride ion. The only source of chloride ion available is a 0.00300 M stock solution of strontium chloride. How much (in L) of this solution is needed for this reaction?arrow_forwardAbsorption Spectrum of NaphthaleneTitle: Understanding the Absorption Spectrum of NaphthaleneGraph: Show a graph with labeled peaks indicating the absorption spectrum of naphthalene in a suitable solventarrow_forwardDon't used Ai solutionarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

