
Concept explainers
Draw structures corresponding to the following names:
(a) 3-Methyl-1, 2-benzenediamine
(b) 1, 3, 5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2, 4, 6-Trinitrophenol (picric acid]
a) 3-methyl-1,2-benzenediamine
Interpretation:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine.
Answer to Problem 19AP
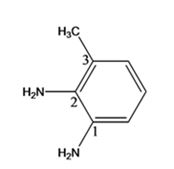
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is
Explanation of Solution
The IUPAC name of the compound, 3-methyl-1,2-benzenediamine, indicates that the compound has a benzene ring with a methyl group on C3 and two amino groups on C1 & C2.
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine

b) 1,3,5-benzenetriol
Interpretation:
The structure of the compound with IUPAC name 1,3,5-benzenetriol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment as carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 1,3,5-benzenetriol.
Answer to Problem 19AP
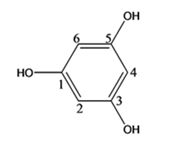
The structure of the compound with IUPAC name 1,3,5-benzenetriol is
Explanation of Solution
The IUPAC name of the compound, 1,3,5-benzenetriol, indicates that the compound has a benzene ring with three hydroxyl groups on C1, C3 & C5.
The structure of the compound with IUPAC name 1,3,5-benzenetriol is

c) 3-methyl-2-phenylhexane
Interpretation:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is to be given.
Concept introduction:
Alkyl-substituted benzenes, called arenes, are named in different ways depending on the size of the alkyl group. If the alkyl group is smaller than the ring (six or fewer carbons), the arene is named as an alkyl-substituted benzene. If the alkyl substituent is larger than the ring (seven or more carbons), the arene is named as a phenyl-substitited alkane.
To draw:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane.
Answer to Problem 19AP
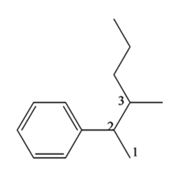
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is
Explanation of Solution
The IUPAC name of the compound, 3-methyl-2-phenyl hexane, indicates that the compound has a six carbon straight chain with a phenyl group on C2 and a methyl group on C3.
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is

d) o-aminobenzoic acid
Interpretation:
The structure of the compound with IUPAC name o-aminobenzoic acid is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta(m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name p-aminobenzoic acid.
Answer to Problem 19AP
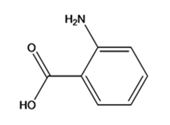
The structure of the compound with IUPAC name p-aminobenzoic acid is
Explanation of Solution
The IUPAC name of the compound, p-aminobenzoic acid, indicates that the compound has a benzene ring with a carboxyl and amino groups in 1,2-relationship.
The structure of the compound with IUPAC name p-aminobenzoicacid is

e) m-bromophenol
Interpretation:
The structure of the compound with IUPAC name m-bromophenol is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name m-bromophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name m-bromophenol is
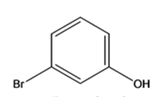
Explanation of Solution
The IUPAC name of the compound, m-bromophenol, indicates that the compound has a benzene ring with a hydroxyl group and bromine atom in 1,3-relationship.
The structure of the compound with IUPAC name m-bromophenol is

f) 2,4,6-trinitrophenol
Interpretation:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is
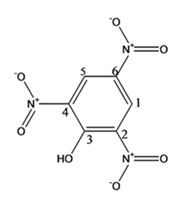
Explanation of Solution
The IUPAC name of the compound, 2,4,6-trinitrophenol, indicates that the compound has a benzenering with a hydroxyl group on C1 and three nitro groups on C2, C4 & C6.
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is

Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- Please correct answer and don't used hand raitingarrow_forward142. A mixture of H2(g) and N2(g) has a density of 0.216 g/liter at 300 K and 500 torr. What is the mole fraction composition of the mixture?arrow_forwardOne liter of N2(g) at 2.1 bar and two liters of Ar(g) at 3.4 bar are mixed in a 4.0 liter flask to form an ideal gas mixture. Calculate the value of the final pressure of the mixture if the initial and final temperature of the gases are the same. Repeat this calculation if the initial temperature of the N2(g) and Ar(g) are 304 K and 402 K, respectively, and the final temperature of the mixture is 377 K.arrow_forward
- 10 5 4. These four 'H NMR spectra were recorded from different isomers with molecular formula CsH,CIO. They all contain a carbonyl group. Determine the structure of the different isomers. 0 10 5 0 10 5 10 9 8 7 6 5 4 3. 1 0 9 10 10 66 9 0 10 9 10 5 1 8 7 6 5 3 2 -a 8 7 6 5 1 10 9 8 7 6 5 22 2 1 0 3 2 16 1 0 3 2 1 2 6 0arrow_forwardUse the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (20.54±0.02 × 0.254±0.003) / (3.21±0.05) = Value: % Error: Absolute error: ± | % (only 1 significant digit) (only 1 significant digit)arrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward
- Nonearrow_forwardWhat functional groups are present in this IRarrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward
- 4) A typical bottle of pop holds carbon dioxide at a pressure of 5 atm. What is the concentration of carbon dioxide in th solution? 5) A stream flowing over rocks and such is exposed to the atmosphere and well aerated. What would be the nitrogen concentration in the water at 25°C? (Air pressure is 1.000 bar.)arrow_forwardUse the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (30.078±0.003) - (20.174±0.001) + (9.813±0.005) = Value: % Error: absolute error: ± % (only 1 significant digit) (only 1 significant digit)arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





