
a)
Interpretation:
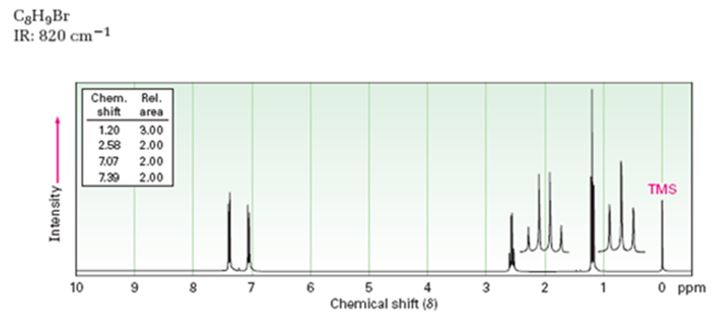
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C8H9Br I.R: 820 cm-1.
1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet).
Concept introduction:
In 1HNMR spectrum
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1 and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C8H9Br I.R: 820 cm-1.
1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet).
Answer to Problem 47AP
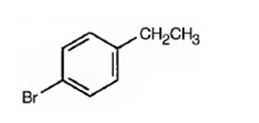
A structure for the compound with M.F = C8H9Br with spectral characteristics: I.R: 820 cm-1, 1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet) is
Explanation of Solution
The molecular formula of the compound is C8H9Br.
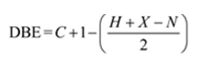
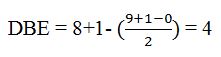
Thus the compound has four unsaturation units like double bonds and/or rings. The two doublets at 7.07δ and 7.39δ each corresponding to two protons indicate the compound is aromatic and each proton responsible for the doublet has a proton on the adjacent carbon. The two proton quartet at 2.58δ (benzylic) and three proton triplet at 1.20δ (primary) could be accounted for only if an ethyl substituent is present on the ring along with bromine. The I.R absorption at 820 cm-1 indicates that the ethyl group and bromine are arranged in para positions. Hence the compound is p-bromoethyl benzene.
A structure for the compound with M.F=C8H9Br with spectral characteristics: I.R: 820 cm-1, 1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet) is

b)
Interpretation:
A structure for the compound whose 1HNMR spectrum given is to be proposed.
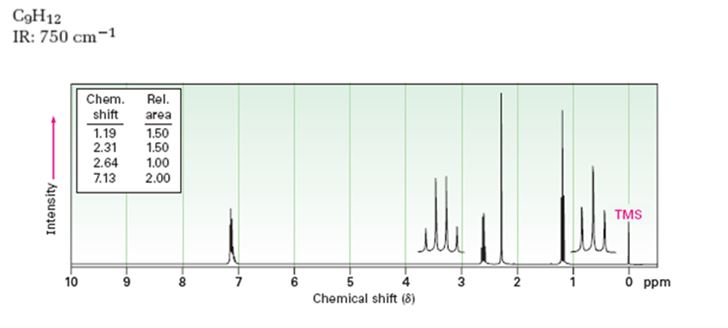
Given: M.F = C9H12 I.R: 750 cm-1
1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet).
Concept introduction:
In 1HNMR spectrum aromatic protons give a broad peak in the range 6.5δ-8.0δ, the primary alkyl protons around 0.7δ-1.3δ, the secondary alkyl protons around 1.2δ-1.6δ, and a tertiary alkyl protons in between 1.4δ-1.8δ. The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12 I.R: 750 cm-1.
1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet).
Answer to Problem 47AP
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12, I.R: 750 cm-1, 1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet) is
Explanation of Solution
The molecular formula of the compound is C9H12.
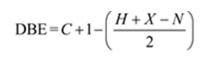
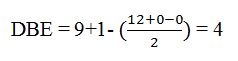
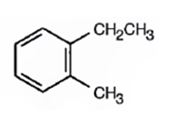
Thus the compound has four unsaturation units like double bonds and/or rings. The four proton broad band at 7.13δ indicates that the compound is aromatic with two substituent groups attached to the benzene ring. The two proton quartet at 2.64δ (benzylic) and three proton triplet at 1.19δ (primary alkyl) could be accounted for only if an ethyl group is present on the ring. The three proton singlet at 2.31δ (benzylic) can be attributed to a methyl group attached to the ring. Thus the two substituent groups attached to the benzene ring are ethyl and methyl. The I.R absorption at 750 cm-1 requires that the ethyl and methyl groups to be arranged in ortho positions. Hence the compound is o-ethyltoluene.
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12, I.R: 750 cm-1, 1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet) is

c)
Interpretation:
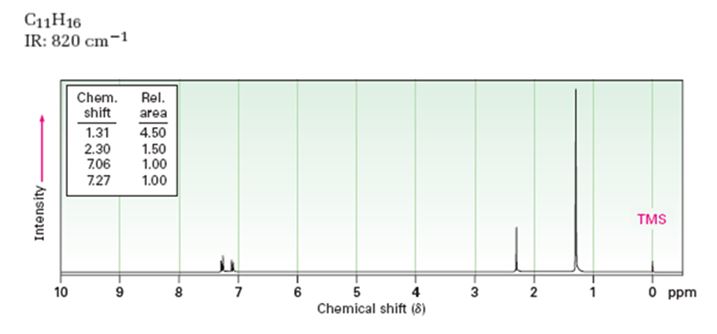
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16 I.R: 820 cm-1.
1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Concept introduction:
In 1HNMR spectrum aromatic protons give a broad peak in the range 6.5δ-8.0δ, the primary alkyl protons around 0.7δ-1.3 δ, the secondary alkyl protons around 1.2δ-1.6δ, and a tertiary alkyl protons in between 1.4δ-1.8δ. The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1 and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16 I.R: 820 cm-1.
1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Answer to Problem 47AP
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16; I.R: 820 cm-1; 1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Explanation of Solution
The molecular formula of the compound is C11H16.
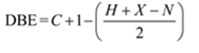
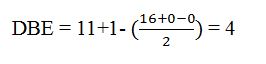
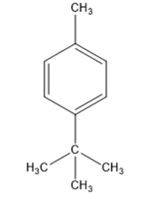
Thus the compound has four unsaturation units like double bonds and/or rings. The two doublets at 7.27δ and 7.06δ each corresponding to two protons indicate the compound is aromatic and each proton responsible for the doublet has a proton on the adjacent carbon. The three proton singlet at 2.58δ (benzylic) could be accounted for a methyl group attached to the benzene ring. The remaining four carbons and nine hydrogens indicate the presence of a tert-butyl group attached to the benzene ring which is confirmed by the nine proton singlet at 1.31δ (primary alkyl). Thus methyl and tert-butyl are the two substituent groups on the benzene ring. The I.R absorption at 820 cm-1 indicates that the two substituent groups are arranged in para positions. Hence the compound is p-t-butyltoluene.
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16; I.R: 820 cm-1; 1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).

Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
- Predict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forward
- Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forward
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


