
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15.3, Problem 15.7KCP
For each compound shown next (a–d), indicate whether the compound is polar or nonpolar, and whether it is soluble or insoluble in water.
(a) 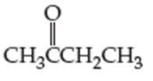
(b) 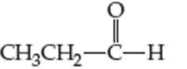
(c) ch3ch2ch2ch2ch3
(d) 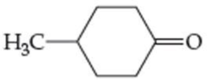
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.
+NH+
CO₂
+P
H₂N
+ ATP
H₂N
NH₂
+ADP
Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.
Chapter 15 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 15.1 - Prob. 15.1PCh. 15.1 - Prob. 15.2PCh. 15.2 - Draw structures corresponding to the following...Ch. 15.2 - Prob. 15.1CIAPCh. 15.2 - Prob. 15.2CIAPCh. 15.2 - Prob. 15.4PCh. 15.2 - Draw the line structures and provide common names...Ch. 15.2 - Prob. 15.6KCPCh. 15.3 - For each compound shown next (ad), indicate...Ch. 15.3 - Prob. 15.8KCP
Ch. 15.4 - Prob. 15.9KCPCh. 15.5 - Indicate whether the following compounds will give...Ch. 15.6 - Draw line structures of the following compounds...Ch. 15.6 - Prob. 15.12PCh. 15.7 - Prob. 15.13PCh. 15.7 - Prob. 15.14PCh. 15.7 - Prob. 15.15PCh. 15.7 - Draw the structure of each acetal or ketal final...Ch. 15.7 - Prob. 15.17PCh. 15.7 - Prob. 15.18PCh. 15.7 - Prob. 15.19PCh. 15.7 - Prob. 15.1MRPCh. 15.7 - Prob. 15.2MRPCh. 15.7 - Prob. 15.3MRPCh. 15.7 - Prob. 15.3CIAPCh. 15.7 - (a) What are mirotubules? (b) Why would drugs that...Ch. 15.7 - Tetrodotoxin, found in the puffer fish, has been...Ch. 15 - Prob. 15.20UKCCh. 15 - Prob. 15.21UKCCh. 15 - Prob. 15.22UKCCh. 15 - Prob. 15.23UKCCh. 15 - Prob. 15.24UKCCh. 15 - Prob. 15.25UKCCh. 15 - ALDEHYDESAND KETONES (SECTIONS 15.1 AND 15.2)...Ch. 15 - Draw a structure for a compound that meets each of...Ch. 15 - Prob. 15.28APCh. 15 - Prob. 15.29APCh. 15 - Draw structures corresponding to the following...Ch. 15 - Draw structures corresponding to the following...Ch. 15 - Prob. 15.32APCh. 15 - Prob. 15.33APCh. 15 - The following names are incorrect. What is wrong...Ch. 15 - Prob. 15.35APCh. 15 - Prob. 15.36APCh. 15 - Prob. 15.37APCh. 15 - Prob. 15.38APCh. 15 - Prob. 15.39APCh. 15 - Prob. 15.40APCh. 15 - Draw the structures of the aldehydes that might be...Ch. 15 - Write the structures of the hemiacetal or...Ch. 15 - Write the structures of the hemiacetal or...Ch. 15 - Prob. 15.44APCh. 15 - Prob. 15.45APCh. 15 - Prob. 15.46APCh. 15 - Prob. 15.47APCh. 15 - Prob. 15.48APCh. 15 - Prob. 15.49APCh. 15 - Prob. 15.50CPCh. 15 - Prob. 15.51CPCh. 15 - Prob. 15.52CPCh. 15 - Prob. 15.53CPCh. 15 - Prob. 15.54CPCh. 15 - Name the following compounds: (a) (b) (c) (be sure...Ch. 15 - Draw the structural formulas of the following...Ch. 15 - Prob. 15.57CPCh. 15 - Complete the following equations (refer to Summary...Ch. 15 - Prob. 15.59CPCh. 15 - How could you differentiate between 3-hexanol and...Ch. 15 - The liquids 1-butanol and butanal have similar...Ch. 15 - Prob. 15.62CPCh. 15 - Prob. 15.63GPCh. 15 - Prob. 15.64GPCh. 15 - Using the ketone structural form of fructose...Ch. 15 - Prob. 15.66GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Which features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forwardLooking at the figure 30-5 what intermolecular forces are present between the substrate and the enzyme and the substrate and cofactors.arrow_forwardprovide short answers to the followings Urgent!arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forward
- Sodium fluoroacetate (FCH 2CO2Na) is a very toxic molecule that is used as rodentpoison. It is converted enzymatically to fluoroacetyl-CoA and is utilized by citratesynthase to generate (2R,3S)-fluorocitrate. The release of this product is a potentinhibitor of the next enzyme in the TCA cycle. Show the mechanism for theproduction of fluorocitrate and explain how this molecule acts as a competitiveinhibitor. Predict the effect on the concentrations of TCA intermediates.arrow_forwardIndicate for the reactions below which type of enzyme and cofactor(s) (if any) wouldbe required to catalyze each reaction shown. 1) Fru-6-P + Ery-4-P <--> GAP + Sed-7-P2) Fru-6-P + Pi <--> Fru-1,6-BP + H2O3) GTP + ADP <--> GDP + ATP4) Sed-7-P + GAP <--> Rib-5-P + Xyl-5-P5) Oxaloacetate + GTP ---> PEP + GDP + CO 26) DHAP + Ery-4-P <--> Sed-1,7-BP + H 2O7) Pyruvate + ATP + HCO3- ---> Oxaloacetate + ADP + Piarrow_forwardTPP is also utilized in transketolase reactions in the PPP. Give a mechanism for theTPP-dependent reaction between Xylulose-5-phosphate and Ribose-5-Phosphate toyield Glyceraldehyde-3-phosphate and Sedoheptulose-7-Phosphate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning



Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning


Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license