
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 15.58CP
Complete the following equations (refer to “Summary of Reactions” in Chapters 13 and 14 if necessary):
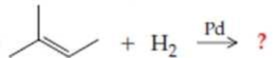
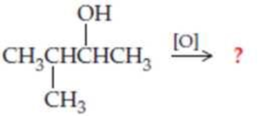
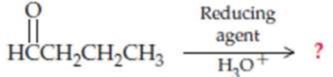
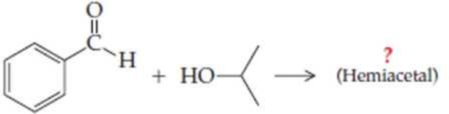
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can koch's postulates of disease causation be applied to non-microorganism disease pathogens (such as molecules)?
Here is my literature Beta Carotene HPLC analysis graph.
Can you help me explain what each peak is at each retention time?
Thank You :D
I have a literature B-Carotene HPLC graph in which showcases a retention time of roughly 23.6 and 25.1.
Please help me compare my two different Anti-Oxidant Juice graphs. (Attached)
The juices provided are: V8 Carrot Ginger Blend and V8 Original Blend
Noticing the HPLC graphs I saw no peaks for the Original Blend for B-Carotene.
However the Carrot Ginger Blend showed similar peaks --> Why is this reason?
Please explain in terms of Retention time and Area (Under Curve).
Thank You!
Chapter 15 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 15.1 - Prob. 15.1PCh. 15.1 - Prob. 15.2PCh. 15.2 - Draw structures corresponding to the following...Ch. 15.2 - Prob. 15.1CIAPCh. 15.2 - Prob. 15.2CIAPCh. 15.2 - Prob. 15.4PCh. 15.2 - Draw the line structures and provide common names...Ch. 15.2 - Prob. 15.6KCPCh. 15.3 - For each compound shown next (ad), indicate...Ch. 15.3 - Prob. 15.8KCP
Ch. 15.4 - Prob. 15.9KCPCh. 15.5 - Indicate whether the following compounds will give...Ch. 15.6 - Draw line structures of the following compounds...Ch. 15.6 - Prob. 15.12PCh. 15.7 - Prob. 15.13PCh. 15.7 - Prob. 15.14PCh. 15.7 - Prob. 15.15PCh. 15.7 - Draw the structure of each acetal or ketal final...Ch. 15.7 - Prob. 15.17PCh. 15.7 - Prob. 15.18PCh. 15.7 - Prob. 15.19PCh. 15.7 - Prob. 15.1MRPCh. 15.7 - Prob. 15.2MRPCh. 15.7 - Prob. 15.3MRPCh. 15.7 - Prob. 15.3CIAPCh. 15.7 - (a) What are mirotubules? (b) Why would drugs that...Ch. 15.7 - Tetrodotoxin, found in the puffer fish, has been...Ch. 15 - Prob. 15.20UKCCh. 15 - Prob. 15.21UKCCh. 15 - Prob. 15.22UKCCh. 15 - Prob. 15.23UKCCh. 15 - Prob. 15.24UKCCh. 15 - Prob. 15.25UKCCh. 15 - ALDEHYDESAND KETONES (SECTIONS 15.1 AND 15.2)...Ch. 15 - Draw a structure for a compound that meets each of...Ch. 15 - Prob. 15.28APCh. 15 - Prob. 15.29APCh. 15 - Draw structures corresponding to the following...Ch. 15 - Draw structures corresponding to the following...Ch. 15 - Prob. 15.32APCh. 15 - Prob. 15.33APCh. 15 - The following names are incorrect. What is wrong...Ch. 15 - Prob. 15.35APCh. 15 - Prob. 15.36APCh. 15 - Prob. 15.37APCh. 15 - Prob. 15.38APCh. 15 - Prob. 15.39APCh. 15 - Prob. 15.40APCh. 15 - Draw the structures of the aldehydes that might be...Ch. 15 - Write the structures of the hemiacetal or...Ch. 15 - Write the structures of the hemiacetal or...Ch. 15 - Prob. 15.44APCh. 15 - Prob. 15.45APCh. 15 - Prob. 15.46APCh. 15 - Prob. 15.47APCh. 15 - Prob. 15.48APCh. 15 - Prob. 15.49APCh. 15 - Prob. 15.50CPCh. 15 - Prob. 15.51CPCh. 15 - Prob. 15.52CPCh. 15 - Prob. 15.53CPCh. 15 - Prob. 15.54CPCh. 15 - Name the following compounds: (a) (b) (c) (be sure...Ch. 15 - Draw the structural formulas of the following...Ch. 15 - Prob. 15.57CPCh. 15 - Complete the following equations (refer to Summary...Ch. 15 - Prob. 15.59CPCh. 15 - How could you differentiate between 3-hexanol and...Ch. 15 - The liquids 1-butanol and butanal have similar...Ch. 15 - Prob. 15.62CPCh. 15 - Prob. 15.63GPCh. 15 - Prob. 15.64GPCh. 15 - Using the ketone structural form of fructose...Ch. 15 - Prob. 15.66GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5arrow_forwardShow a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forward
- Don't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forwardhow would you make this plot in excel?arrow_forwardwhat is the productarrow_forward
- Balance the following equation and list of coefficients in order from left to right. SF4+H2O+—-> H2SO3+HFarrow_forwardProblem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:CengageCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:CengageCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage




Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Essentials of Pharmacology for Health Professions
Nursing
ISBN:9781305441620
Author:WOODROW
Publisher:Cengage

Case Studies In Health Information Management
Biology
ISBN:9781337676908
Author:SCHNERING
Publisher:Cengage
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license