
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
12th Edition
ISBN: 9781337915977
Author: Bettelheim
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 8P
16-13 Classify each amino group as primary, secondary, or tertiary and as aliphatic or
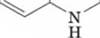
Serotonin
(a neurotransmitter)
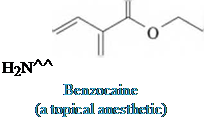
Diphenhydramine
(the hydrochloride salt is
the antihistamine Benadryl)
Lysine
(an amino acid)
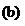
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write the chemical equation for a pentanoate ion acting as base when it reacts with hydrochloric acid (HCI).
Which is formula of this rule of reaction?
True or false
The hydroxyl group found in an organic compound is responsible for its basicity.
Aromatic hydrocarbons are considered polar and has high affinity to water.
Acidic drugs are a class of chemical compounds that normally have high hydrophilicity and negative charges.
Amino groups attached to hydrocarbons are considered polar which may be responsible for its solubility.
Lipophilic drugs have a faster rate of absorption than hydrophilic drugs.
Complete this table for different amine compounds.
Chemical
propylamine
quaternary
ammonium ion
methylphenylamine
Molecular
formula
C3H9N
C₂H7N
C5H13N
C4H10N
Structural formula
(CH3)3N
(CH3)2NH
CH 3
I
(CH₂) 11
|
H3C(CH2) 11 -N-(CH₂)11CH3
NHCH3
(CH₂)11
CH3
CH3CH2CH2-NH-CH3
+
Classification
Tertiary
Quaternary
Tertiary
Secondary
Chapter 15 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
Ch. 15.1 - Prob. 15.1QCCh. 15.2 - Problem 16-2 Write a structural formula for each...Ch. 15.2 - Prob. 15.3QCCh. 15.3 - Problem 16-4 Select the stronger base from each...Ch. 15.3 - Prob. 15.5QCCh. 15 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 15 - Prob. 2PCh. 15 - Prob. 3PCh. 15 - 16-9 In what way are pyridine and pyrimidine...Ch. 15 - Prob. 5P
Ch. 15 - Prob. 6PCh. 15 - Prob. 7PCh. 15 - 16-13 Classify each amino group as primary,...Ch. 15 - Prob. 9PCh. 15 - 16-15 There are eight primary amines with the...Ch. 15 - Prob. 11PCh. 15 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 15 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 15 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 15 - Prob. 15PCh. 15 - Prob. 16PCh. 15 - Prob. 17PCh. 15 - Prob. 18PCh. 15 - Prob. 19PCh. 15 - Prob. 20PCh. 15 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 15 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 15 - 16-28 Following is the structural formula of...Ch. 15 - Prob. 24PCh. 15 - Prob. 25PCh. 15 - Prob. 26PCh. 15 - 16*32 Many tumors of the breast are correlated...Ch. 15 - Prob. 28PCh. 15 - Prob. 29PCh. 15 - Prob. 30PCh. 15 - (Chemical Connections 15B ) Identify all...Ch. 15 - Prob. 32PCh. 15 - Prob. 33PCh. 15 - Prob. 34PCh. 15 - Prob. 35PCh. 15 - Prob. 36PCh. 15 - (Chemical Connections 15D ) Suppose you saw this...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - 16-46 Arrange these three compounds in order of...Ch. 15 - Prob. 42PCh. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - Prob. 47PCh. 15 - Prob. 48PCh. 15 - 16-54 Several poisonous plants, including Atropa...Ch. 15 - Prob. 50PCh. 15 - Prob. 51PCh. 15 - Prob. 52PCh. 15 - 16-58 Following is a structural formula of...Ch. 15 - Prob. 54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16-54 Several poisonous plants, including Atropa belladonna, contain the alkaloid atropine. The name “belladonna” (which means “beautiful lady”) probably comes from the fact that Roman women used extracts from this plant to make themselves more attractive. Atropine is widely used by ophthal mologists and optometrists to dilate the pupils for eye examination. Classify the amino group in atropine as primary, secondary, or tertiary. Locate all stereocenters in atropine. Account for the fact that atropine is almost insoluble in water (1 g in 455 mL of cold water) but atropine hydrogen sulfate is very soluble (1 g in 5 mL of cold water). Account for the fact that a dilute aqueous solution of atropine is basic (pH approximately 10.0).arrow_forward16-28 Following is the structural formula of metformin, the hydrochloride salt of which is marketed as the antidiabetic medication Glucophage. Metformin was introduced into clinical practice in the United States in 1995 for the treatment of type 2 diabetes. More than 25 million prescriptions for this drug were written in 2000, making it the most commonly prescribed brand-name diabetes medication in the nation. NH NH H3(\ 3 N N Nh2ch3 h Metformin Complete the Lewis structure for metformin, showing all valence electrons. Which nitrogen is the most likely site of protonation? Draw the structural formula of Glucophage.arrow_forward17-13 Which compounds contain carbonyl groups?arrow_forward
- 18-47 Methylparaben and propylparaben are used as preservatives in foods, beverages, and cosmetics. Show how each of these preservatives can be prepared from 4-aminobenzoic acid.arrow_forward13-27 Define autoxidation.arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward
- 16-15 There are eight primary amines with the molecular formula C5H13N. (a) Name and draw a structural formula for each amine. (b > Which of these amines are chiral?arrow_forward18-19 The following compounds have approximately the same molecular weight: hexanoic acid, heptanal, and 1-heptanol. Arrange them in order of increasing boiling point.arrow_forward16-18 Account for the fact that 1-butanamine (bp 78°C) has a lower boiling point than 1-butanol (bp 117°C)arrow_forward
- 16-6 Answer true or false. te/7-Butylamine is a 3° amine. In an aromatic amine, one or more of the groups bonded to nitrogen is an aromatic ring. In a heterocyclic amine, the amine nitrogen is one of the atoms of a ring. The Lewis structures of both NH4~ and CH4show the same number (eight) of valence electrons, and the VSEPR model predicts tetrahedral geometry for each. There are four constitutional isomers with the molecular formula CgH^N.arrow_forward18-29 Complete the equations for these acid—base reactions. (a) (b) (c) (d) (e)arrow_forward18-30 Complete the equations for these acid-base reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY