
Concept explainers
16-6 Answer true or false.
- te/7-Butylamine is a 3°
amine .
(a)
Interpretation:
To analyse whether the given statement: tert-Butylamine is
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is false.
Explanation of Solution
The structure of a tert-butylamine is given below:
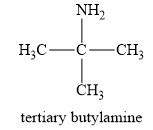
In a tert-butylamine, the carbon atom is bonded to three methyl groups, while nitrogen is attached to only one carbon group. Therefore, the tert-butyl amine is a primary amine. Therefore, this statement is false.
(b)
Interpretation:
To analyse whether the given statement about the aromatic amine is true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is true.
Explanation of Solution
If in an amine, the nitrogen is directly bonded to one or more aryl groups or aromatic rings, and then the amine is known as an aromatic amine.
For example, aniline is an aromatic amine, as it is attached to one aromatic ring benzene. The structure of aniline is given below:
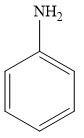
Therefore, the given statement is true.
(c)
Interpretation:
To analyse whether the given statement: In a heterocyclic amine, the main nitrogen is the part of the ringis true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is true.
Explanation of Solution
In a heterocyclic amine, the carbon group of the ring structure is replaced by the nitrogen atom. For example, in pyridine, the nitrogen atom replaces one —CH group of the benzene ring.
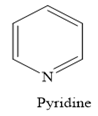
Thus, the heterocyclic amine is an amine in which nitrogen is one of the atoms of a ring. Therefore, this statement is true.
(d)
Interpretation:
To analyze whether the given statement: The NH4+ and CH4have the same number of valence electrons and both have tetrahedral geometry as per the VSEPR model is true or not.
Concept Introduction:
The VSEPR (Valence-shell electron pair repulsion) model predicts the shape of the molecules or ions by identifying the position of atoms connected to the central atom.
Answer to Problem 1P
The statement is true.
Explanation of Solution
The valence shell electron pair repulsion (VSEPR) theory determines the shapes and the geometry of a molecule. In a CH4 molecule, one carbon atom is bonded to four hydrogen atoms with a single bond. In NH4+, one nitrogen atom is singly bonded to four hydrogen atoms.
According to the VSEPR model, each bond represents a pair of electrons. Since both compounds have four bonds around the central atom, they contain eight valence electrons. Also, according to the VSEPR model, these four bonding regions are arranged in a tetrahedral manner so that they are as far away from one another as possible, giving the molecule a tetrahedral geometry. Therefore, this statement is true.
(e)
Interpretation:
To analyze whether the given statement: There are four constitutional isomers with the molecular formulaC3H9N, is true or not.
Concept Introduction:
Constitutional isomers are those compounds which have same molecular formula, but they differ in the arrangement of atoms in the molecules.
Answer to Problem 1P
The statement is true.
Explanation of Solution
The molecular formula is given as C3H9N. Therefore, the possible constitutional isomers for this molecular formula are given below:
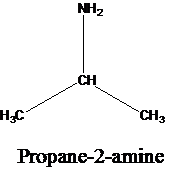
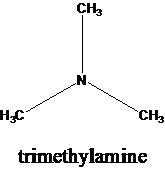
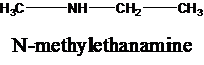
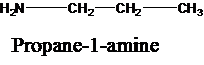
Thus, there are four constitutional isomers possible with the molecular formula C3H9N. Therefore, this statement is true.
Want to see more full solutions like this?
Chapter 15 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- Dr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forwardExperiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forward
- Illustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forward
- CS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning





