
Concept explainers
(a)
Interpretation:
To check whether the statement, “Aqueous solutions of
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

From the reaction, it is clear that the nitrogen donates a pair of electrons to hydrogen thus, making a salt. Thus, aqueous solutions of amines are basic in nature.
Hence, the statement is True.
(b)
Interpretation:
To check whether the statement, “
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
Aniline is the benzene ring that is attached to amine group. The lone pair of electrons present on the nitrogen atom is in conjugation with the aromatic ring. Thus, the lone pair of electrons is less available to donate to a proton. Hence, aniline is less basic in nature in comparison with methyl amine.
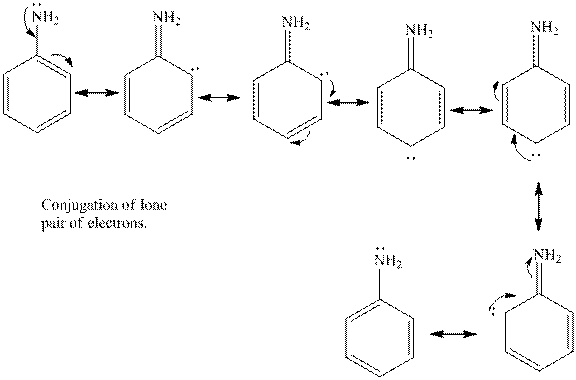
The cyclohexylamine is the one where the cyclohexyl ring is attached to the amine group. Hence, it is similar to an alkyl group attached to amine group. The alkyl groups are electron donating in nature due to inductive effect. Hence, the basicity of the amine nitrogen increases due to the attached cyclohexyl ring. Hence, the compound is more basic.
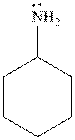
Thus, cyclohexylamine is more basic than aniline. Hence, aromatic amines are weaker bases than aliphatic amines.
The given statement is True.
(c)
Interpretation:
To check whether the statement, “Aliphatic amines are stronger bases than inorganic bases such as NaOH and KOH” is true or false.
Concept Introduction:
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
Explanation of Solution
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
NaOH and KOH are the acids which readily give hydroxide ions when added to water. They dissociate completely. The reaction is not reversible and hence, they are strong acids.
Aliphatic amines with general formula

Hence, the statement is False.
(d)
Interpretation:
To check whether the statement, “Water insoluble amines react with strong aqueous acids such as HCl to form water soluble salts” is true or false.
Concept Introduction:
Water has a property to ionize salts. This property helps in dissolution of salts in water.
Explanation of Solution
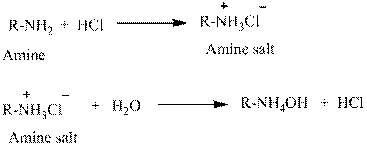
Water is an universal solvent. Its polarity and many other aspects make it an unique solvent. It also has the property to ionize. This property makes salts to be easily dissolved in water. For this reason, amines which are usually insoluble in water are made soluble by converting into hydrochloride salts.
(e)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 2.0 by the addition of concentrated HCl, the amine will be present in solution almost entirely as its conjugate acid” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 2.0. That means, it is on the acidic side. As the acidic environment is present, the species that will present is the amine salt.
Hence, the statement is True.
(f)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 10.0 by the addition of NaOH, the amine will be present in solution almost entirely as the free base” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 10.0 by addition of NaOH. That means, the pH is on the basic side which is because of the added NaOH. As the basic environment is present, the species that will present is the basic amine.
Hence, the statement is True.
(g)
Interpretation:
To check whether the statement, “For a primary amine, the concentrations of salt and basic amine will be equal when the pH of the solution is equal to the pKb of the amine” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. At certain pH such as pKb the system is at equilibrium. pKb is the equilibrium dissociation constant pH. That means, the pH is so supporting that the forward and reverse reactions are at equal rate. That means, there will be forward and reverse reactions but that is not noticeable because they are at equal rate.
Hence, at this pH, the concentrations of salt and the basic salt will be equal. Hence, the statement is True.
Want to see more full solutions like this?
Chapter 15 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forwardUsing iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





