
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 71QAP
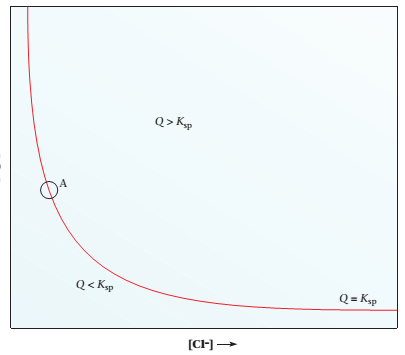
Consider the equilibrium curve for AgCl shown below.
Which of the following statements about a solution at point A on the curve are true?
(a) The solution is saturated and at equilibrium.
(b) Addition of NaCl increases the concentration of Cl- in solution.
(c) Addition of NaCl increases the concentration of in solution.
(d) Addition of Ag+ results in the precipitation of AgCl.
(e) Addition of solid NaNO3 to the solution without change in volume does not change [Ag+] or [Cl-].
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that:
(from Box 5.1, pg. 88 of your text):
Temp = 18°C
Salinity = 35 ppt
Density = 1025 kg/m3
Oxygen concentration measured in bulk water = 263.84 mmol/m3
Wind speed = 7.4 m/s
Oxygen is observed to be about 10% initially supersaturated
What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?
Find a molecular formula for these unknowns
(ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.
Chapter 15 Solutions
Chemistry: Principles and Reactions
Ch. 15 - Write a net ionic equation for the reaction...Ch. 15 - Write a net ionic equation for the reaction...Ch. 15 - Write a balanced net ionic equation for the...Ch. 15 - Write a balanced net ionic equation for the...Ch. 15 - Calculate K for the reactions in Question 1.Ch. 15 - Calculate K for the reactions in Question 2.Ch. 15 - Calculate K for the reactions in Question 3.Ch. 15 - Calculate K for the reactions in Question 4.Ch. 15 - Calculate [H+] and pH in a solution in which...Ch. 15 - Calculate [OH-] and pH in a solution in which the...
Ch. 15 - A buffer is prepared by dissolving 0.0250 mol of...Ch. 15 - Prob. 12QAPCh. 15 - A buffer solution is prepared by adding 15.00 g of...Ch. 15 - A buffer solution is prepared by adding 5.50 g of...Ch. 15 - A solution with a pH of 9.22 is prepared by adding...Ch. 15 - An aqueous solution of 0.057 M weak acid, HX, has...Ch. 15 - Which of the following would form a buffer if...Ch. 15 - Which of the following would form a buffer if...Ch. 15 - Calculate the solubility (in grams per liter) of...Ch. 15 - Calculate the solubility (in grams per liter) of...Ch. 15 - Copper(l) chloride, CuCl, is the starting material...Ch. 15 - Prob. 22QAPCh. 15 - Prob. 23QAPCh. 15 - Ksp for CaSO4 at 100C is estimated to be1.6105. At...Ch. 15 - Prob. 25QAPCh. 15 - At 25C, 10.24 mg of Cr(OH)2 are dissolved in...Ch. 15 - Calcium nitrate is added to a sodium sulfate...Ch. 15 - Cadmium(ll) chloride is added to a solution of...Ch. 15 - Water from a well is found to contain 3.0 mg of...Ch. 15 - Silver(I) sulfate (Ksp=1.2105) is used in the...Ch. 15 - A solution is prepared by mixing 13.00 mL of...Ch. 15 - A solution is prepared by mixing 45.00 mL of 0.022...Ch. 15 - A solution is 0.047 M in both NaF and Na2CO3....Ch. 15 - Solid lead nitrate is added to a solution that is...Ch. 15 - A solution is made up by adding 0.632 g of barium...Ch. 15 - A solution is made up by adding 0.839 g of...Ch. 15 - Prob. 37QAPCh. 15 - To a beaker with 500 mL of water are added 95 mg...Ch. 15 - Write net ionic equations for the reaction of H+...Ch. 15 - Prob. 40QAPCh. 15 - Prob. 41QAPCh. 15 - Write a net ionic equation for the reaction with...Ch. 15 - Prob. 43QAPCh. 15 - Write a net ionic equation for the reaction with...Ch. 15 - Prob. 45QAPCh. 15 - Write an overall net ionic equation and calculate...Ch. 15 - Consider the reaction...Ch. 15 - Consider the reaction BaF2(s)+SO42(aq)BaSO4(s)+2...Ch. 15 - Aluminum hydroxide reacts with an excess of...Ch. 15 - Prob. 50QAPCh. 15 - Calculate the molar solubility of gold(I) chloride...Ch. 15 - Calculate the molar solubility of PbCl2 in 0.2 M...Ch. 15 - For the reaction...Ch. 15 - For the reaction Zn(OH)2(s)+2OH(aq)Zn(OH)42(aq)...Ch. 15 - What are the concentrations of Cu2+, NH3, and...Ch. 15 - Prob. 56QAPCh. 15 - Calcium ions in blood trigger clotting. To prevent...Ch. 15 - Prob. 58QAPCh. 15 - A town adds 2.0 ppm of F- ion to fluoridate its...Ch. 15 - Consider the following hypothetical dissociation:...Ch. 15 - Prob. 61QAPCh. 15 - Consider a 2.0-L aqueous solution of 4.17 M NH3,...Ch. 15 - Marble is almost pure CaCO3. Acid rain has a...Ch. 15 - Prob. 64QAPCh. 15 - Prob. 65QAPCh. 15 - The box below represents one liter of a saturated...Ch. 15 - Consider a saturated solution of BaCO3 at 7.5C....Ch. 15 - Prob. 68QAPCh. 15 - Consider the insoluble salts JQ, K2R, L2S3, MT2,...Ch. 15 - Prob. 70QAPCh. 15 - Consider the equilibrium curve for AgCl shown...Ch. 15 - Dissolving CaCO3 is an endothermic reaction. The...Ch. 15 - Challenge Problems Insoluble hydroxides such as...Ch. 15 - What is the solubility of CaF2 in a buffer...Ch. 15 - What is [Br-] just as AgCl begins to precipitate...Ch. 15 - Prob. 76QAPCh. 15 - Prob. 77QAPCh. 15 - Prob. 78QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardThe following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward
- Find a molecular formula. ( MW: 102 )arrow_forwardExperiment #8 Electrical conductivity & Electrolytes. Conductivity of solutions FLINN Scientific Scale RED LED Green LED LED Conductivity 0 OFF OFF 1 Dim OFF 2 medium OFF 3 Bright Dim Low or Nowe Low Medium High 4 Very Bright Medium nd very high AA Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ SE=Strong Electrolyte, FE = Fair Electrolyte CWE = Weak Electrolyte, NE= Noni Electrolyte, #Solutions 1 0.1 M NaCl 2/1x 102 M NaCl, 3/1X103 M Nall Can Prediction M Observed Conductivity Very bright red Bright red Dim red you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAA Darrow_forward(SE EX 2) Prblsm 4-7: Can you please explain problems 4-7 and color code if needed for me. (step by step) detail explanationsarrow_forward
- (SE EX 2) Problems 8-11, can you please explain them to me in detail and color-code anything if necessary?arrow_forward(ME EX2) Problems 15-16 Could you please explain problems 15 through 16 to me in detail, step by step? Thank you so much! If necessary, please color-code them for me.arrow_forward1.)show any electrophilic aromatic substitution, identify the electriphile, nucleophile and transition statearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY