
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 66QAP
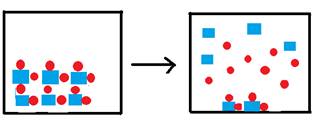
The box below represents one liter of a saturated solution of the species 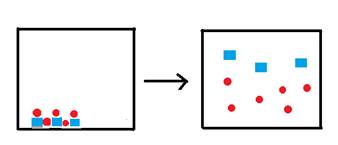 , where squares represent the cation and circles represent the anion. Water molecules, though present, are not shown.
, where squares represent the cation and circles represent the anion. Water molecules, though present, are not shown.
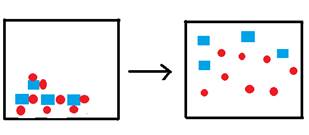
Complete the next three figures below by filling one-liter boxes to the right of the arrow, showing the state of the ions after water is added to form saturated solutions. The species represented to the left of the arrow is the solid form of the ions represented above. Do not show the water molecules.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. Complete the following halogenation reactions for alkanes. Draw the structures of one of the
many possible products for each reaction. Name the reactant and product.
a)
CH₂- CH-CH2-CH3 + Br₂
CH₂
UV
UV
b)
+ Cl2
c)
CH3-CH₂
CHICHCHICH-CH
CH₂-CH₂
+ F2
UV
Which of the following processes involves the largest photon energy?
Group of answer choices
Electron promotion from n=2 to n=5
Electron relaxing from n=4 to n=3
Ionization of an electron from n=2
Ionization of an electron from n=4
Which of the following compounds does not match atomic ratio expectations in Mendeleev's 1872 periodic table?
Group of answer choices
NO2
Al2O3
SO3
CaO
Chapter 15 Solutions
Chemistry: Principles and Reactions
Ch. 15 - Write a net ionic equation for the reaction...Ch. 15 - Write a net ionic equation for the reaction...Ch. 15 - Write a balanced net ionic equation for the...Ch. 15 - Write a balanced net ionic equation for the...Ch. 15 - Calculate K for the reactions in Question 1.Ch. 15 - Calculate K for the reactions in Question 2.Ch. 15 - Calculate K for the reactions in Question 3.Ch. 15 - Calculate K for the reactions in Question 4.Ch. 15 - Calculate [H+] and pH in a solution in which...Ch. 15 - Calculate [OH-] and pH in a solution in which the...
Ch. 15 - A buffer is prepared by dissolving 0.0250 mol of...Ch. 15 - Prob. 12QAPCh. 15 - A buffer solution is prepared by adding 15.00 g of...Ch. 15 - A buffer solution is prepared by adding 5.50 g of...Ch. 15 - A solution with a pH of 9.22 is prepared by adding...Ch. 15 - An aqueous solution of 0.057 M weak acid, HX, has...Ch. 15 - Which of the following would form a buffer if...Ch. 15 - Which of the following would form a buffer if...Ch. 15 - Calculate the solubility (in grams per liter) of...Ch. 15 - Calculate the solubility (in grams per liter) of...Ch. 15 - Copper(l) chloride, CuCl, is the starting material...Ch. 15 - Prob. 22QAPCh. 15 - Prob. 23QAPCh. 15 - Ksp for CaSO4 at 100C is estimated to be1.6105. At...Ch. 15 - Prob. 25QAPCh. 15 - At 25C, 10.24 mg of Cr(OH)2 are dissolved in...Ch. 15 - Calcium nitrate is added to a sodium sulfate...Ch. 15 - Cadmium(ll) chloride is added to a solution of...Ch. 15 - Water from a well is found to contain 3.0 mg of...Ch. 15 - Silver(I) sulfate (Ksp=1.2105) is used in the...Ch. 15 - A solution is prepared by mixing 13.00 mL of...Ch. 15 - A solution is prepared by mixing 45.00 mL of 0.022...Ch. 15 - A solution is 0.047 M in both NaF and Na2CO3....Ch. 15 - Solid lead nitrate is added to a solution that is...Ch. 15 - A solution is made up by adding 0.632 g of barium...Ch. 15 - A solution is made up by adding 0.839 g of...Ch. 15 - Prob. 37QAPCh. 15 - To a beaker with 500 mL of water are added 95 mg...Ch. 15 - Write net ionic equations for the reaction of H+...Ch. 15 - Prob. 40QAPCh. 15 - Prob. 41QAPCh. 15 - Write a net ionic equation for the reaction with...Ch. 15 - Prob. 43QAPCh. 15 - Write a net ionic equation for the reaction with...Ch. 15 - Prob. 45QAPCh. 15 - Write an overall net ionic equation and calculate...Ch. 15 - Consider the reaction...Ch. 15 - Consider the reaction BaF2(s)+SO42(aq)BaSO4(s)+2...Ch. 15 - Aluminum hydroxide reacts with an excess of...Ch. 15 - Prob. 50QAPCh. 15 - Calculate the molar solubility of gold(I) chloride...Ch. 15 - Calculate the molar solubility of PbCl2 in 0.2 M...Ch. 15 - For the reaction...Ch. 15 - For the reaction Zn(OH)2(s)+2OH(aq)Zn(OH)42(aq)...Ch. 15 - What are the concentrations of Cu2+, NH3, and...Ch. 15 - Prob. 56QAPCh. 15 - Calcium ions in blood trigger clotting. To prevent...Ch. 15 - Prob. 58QAPCh. 15 - A town adds 2.0 ppm of F- ion to fluoridate its...Ch. 15 - Consider the following hypothetical dissociation:...Ch. 15 - Prob. 61QAPCh. 15 - Consider a 2.0-L aqueous solution of 4.17 M NH3,...Ch. 15 - Marble is almost pure CaCO3. Acid rain has a...Ch. 15 - Prob. 64QAPCh. 15 - Prob. 65QAPCh. 15 - The box below represents one liter of a saturated...Ch. 15 - Consider a saturated solution of BaCO3 at 7.5C....Ch. 15 - Prob. 68QAPCh. 15 - Consider the insoluble salts JQ, K2R, L2S3, MT2,...Ch. 15 - Prob. 70QAPCh. 15 - Consider the equilibrium curve for AgCl shown...Ch. 15 - Dissolving CaCO3 is an endothermic reaction. The...Ch. 15 - Challenge Problems Insoluble hydroxides such as...Ch. 15 - What is the solubility of CaF2 in a buffer...Ch. 15 - What is [Br-] just as AgCl begins to precipitate...Ch. 15 - Prob. 76QAPCh. 15 - Prob. 77QAPCh. 15 - Prob. 78QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Need help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forwardWhich of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forward
- Give the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forwardCalculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forward
- How is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forwardPart II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY