
Concept explainers
(a)
Interpretation:
To predict the compound formed by the reaction of phosgene with given compound.
Concept introduction:
Carbonyl carbon of phosgene is highly polar in nature because of the presence of electronegative groups attached to it. So phosgene will undergo nucleophilic addition-eliminationreaction. Tetrahedral intermediate is formed when nucleophile is added to the carbonyl carbon of phosgene. The tetrahedral intermediate is unstable therefore chloride ion gets eliminated. The acyl chloride reacts with neutral nucleophile by the following mechanism:
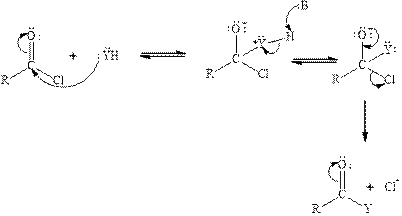
(b)
Interpretation:
To predict the compound formed by the reaction of phosgene with given compound.
Concept introduction:
Carbonyl carbon of phosgene is highly polar in nature because of the presence of electronegative groups attached to it. So phosgene will undergo nucleophilic addition-eliminationreaction. Tetrahedral intermediate is formed when nucleophile is added to the carbonyl carbon of phosgene. The tetrahedral intermediate is unstable therefore chloride ion gets eliminated. The acyl chloride reacts with neutral nucleophile by the following mechanism:

(c)
Interpretation:
To predict the compound formed by the reaction of phosgene with given compound.
Concept introduction:
Carbonyl carbon of phosgene is highly polar in nature because of the presence of electronegative groups attached to it. So phosgene will undergo nucleophilic addition-eliminationreaction. Tetrahedral intermediate is formed when nucleophile is added to the carbonyl carbon of phosgene. The tetrahedral intermediate is unstable therefore chloride ion gets eliminated. The acyl chloride reacts with neutral nucleophile by the following mechanism:

(d)
Interpretation:
To predict the compound formed by the reaction of phosgene with given compound.
Concept introduction:
Carbonyl carbon of phosgene is highly polar in nature because of the presence of electronegative groups attached to it. So phosgene will undergo nucleophilic addition-eliminationreaction. Tetrahedral intermediate is formed when nucleophile is added to the carbonyl carbon of phosgene. The tetrahedral intermediate is unstable therefore chloride ion gets eliminated. The acyl chloride reacts with neutral nucleophile by the following mechanism:

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





