
(a)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with
Concept introduction:
Esters can be prepared from the reaction of an alcohol with
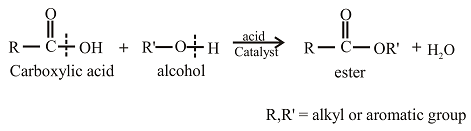
The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
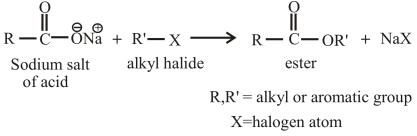
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,
(b)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(c)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(d)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


