
Concept explainers
(a)
Interpretation:
Three-dimensional representation of a given Fischer projection should be drawn.
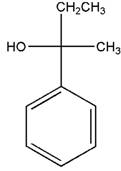
Concept Introduction:
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
(b)
Interpretation:
Three-dimensional representation of a given Fischer projection should be drawn.
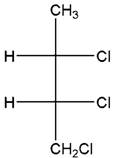
Concept Introduction:
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
General, Organic, & Biological Chemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


