
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15.3, Problem 15.10P
Interpretation Introduction
(a)
Interpretation:
The chiral center in following compound should be located and both the enantiomers should be drawn:
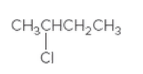
Concept Introduction:
Compounds containing four different groups attached to them are known as chiral compound and that carbon is known as chiral carbon.
Interpretation Introduction
(b)
Interpretation:
The chiral center in following compound should be located and both the enantiomers should be drawn:
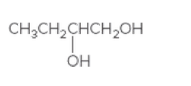
Concept Introduction:
Compounds containing four different groups attached to them are known as chiral compound and that carbon is known as chiral carbon.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Chapter 15 Solutions
General, Organic, & Biological Chemistry
Ch. 15.1 - Prob. 15.1PCh. 15.1 - For trans-2-hexene: (a) draw a stereoisomer; (b)...Ch. 15.2 - Prob. 15.3PCh. 15.2 - Prob. 15.4PCh. 15.3 - Prob. 15.5PCh. 15.3 - Prob. 15.6PCh. 15.3 - Prob. 15.7PCh. 15.3 - Prob. 15.8PCh. 15.3 - Prob. 15.9PCh. 15.3 - Prob. 15.10P
Ch. 15.3 - Prob. 15.11PCh. 15.4 - Prob. 15.12PCh. 15.4 - Prob. 15.13PCh. 15.5 - Prob. 15.14PCh. 15.6 - Prob. 15.15PCh. 15.6 - Prob. 15.16PCh. 15.6 - Prob. 15.17PCh. 15.7 - Prob. 15.18PCh. 15.7 - Prob. 15.19PCh. 15.7 - Prob. 15.20PCh. 15.7 - Prob. 15.21PCh. 15.8 - Prob. 15.22PCh. 15.8 - Prob. 15.23PCh. 15.9 - Prob. 15.24PCh. 15 - Prob. 15.25PCh. 15 - Prob. 15.26PCh. 15 - Prob. 15.27PCh. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - Prob. 15.35PCh. 15 - Prob. 15.36PCh. 15 - Prob. 15.37PCh. 15 - Prob. 15.38PCh. 15 - Prob. 15.39PCh. 15 - Prob. 15.40PCh. 15 - How are the compounds in each pair related? Are...Ch. 15 - Prob. 15.42PCh. 15 - Prob. 15.43PCh. 15 - Prob. 15.44PCh. 15 - Answer each question with a compound of molecular...Ch. 15 - Prob. 15.46PCh. 15 - Prob. 15.47PCh. 15 - Prob. 15.48PCh. 15 - Prob. 15.49PCh. 15 - Prob. 15.50PCh. 15 - Prob. 15.51PCh. 15 - Prob. 15.52PCh. 15 - Prob. 15.53PCh. 15 - Prob. 15.54PCh. 15 - Prob. 15.55PCh. 15 - Prob. 15.56PCh. 15 - (a) Define the terms “optically active” and...Ch. 15 - Prob. 15.58PCh. 15 - Prob. 15.59PCh. 15 - Prob. 15.60PCh. 15 - Prob. 15.61PCh. 15 - Prob. 15.62PCh. 15 - Prob. 15.63PCh. 15 - Prob. 15.64PCh. 15 - Prob. 15.65PCh. 15 - Prob. 15.66PCh. 15 - Prob. 15.67PCh. 15 - Prob. 15.68PCh. 15 - Prob. 15.69PCh. 15 - Prob. 15.70PCh. 15 - Prob. 15.71PCh. 15 - Prob. 15.72PCh. 15 - Prob. 15.73CPCh. 15 - Prob. 15.74CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
