
(a)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
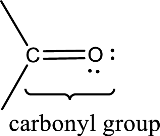
(a)
Answer to Problem 15.1EP
Carbonyl group is present in this compound.
Explanation of Solution
Given compound is,
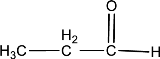
In the above structure, a double bond is present between the carbon and oxygen atom. This is the carbonyl group.
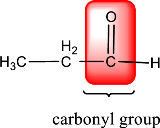
Therefore, the compound that is drawn above has a carbonyl group in its structure.
The given compound contains a carbonyl group in its structure.
(b)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
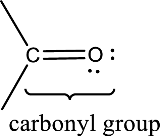
(b)
Answer to Problem 15.1EP
Carbonyl group is not present in this compound.
Explanation of Solution
Given compound is,
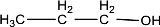
In the above structure, no double bond is present between the carbon and oxygen atom. Therefore, the compound that is given does not have a carbonyl group in its structure.
The given compound does not contain a carbonyl group in its structure.
(c)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
(c)
Answer to Problem 15.1EP
Carbonyl group is not present in this compound.
Explanation of Solution
Given compound is,

In the above structure, no double bond is present between the carbon and oxygen atom. Therefore, the compound that is given does not have a carbonyl group in its structure.
The given compound does not contain a carbonyl group in its structure.
(d)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
(d)
Answer to Problem 15.1EP
Carbonyl group is present in this compound.
Explanation of Solution
Given compound is,
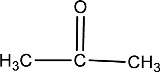
In the above structure, a double bond is present between the carbon and oxygen atom. This is the carbonyl group.
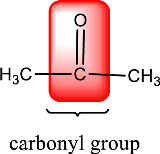
Therefore, the compound that is drawn above has a carbonyl group in its structure.
The given compound contains a carbonyl group in its structure.
Want to see more full solutions like this?
Chapter 15 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- please draw in the answeres, thank youarrow_forwardA) What is being shown here?B) What is indicated by the RED arrow?C) What is indicated by the BLUE arrow?arrow_forwardPlease identify the curve shown below. What does this curve represent? Please identify A, B, C, D, and E (the orange oval). What is occurring in these regions?arrow_forward
- Please identify the test shown here. 1) What is the test? 2) What does the test indicate? How is it performed? What is CX? 3) Why might the test be performed in a clinical setting? GEN CZ CX CPZ PTZ CACarrow_forwardDetermine how much ATP would a cell produce when using fermentation of a 50 mM glucose solution?arrow_forwardDetermine how much ATP would a cell produce when using aerobic respiration of a 7 mM glucose solution?arrow_forward
- Determine how much ATP would a cell produce when using aerobic respiration to degrade one small protein molecule into 12 molecules of malic acid, how many ATP would that cell make? Malic acid is an intermediate in the Krebs cycle. Assume there is no other carbon source and no acetyl-CoA.arrow_forwardIdentify each of the major endocrine glandsarrow_forwardCome up with a few questions and answers for umbrella species, keystone species, redunant species, and aquatic keystone speciesarrow_forward
- 19. On the diagram below a. Label the three pictures as: DNA; polypeptide; or RNA. b. Label the arrows as: translation or transcription/RNA processing. c. Add the following details to the diagram. Promoter region TATA box Transcription start site Transcription terminator Intron (A,B,C,D) Exons (1,2,3,4,5) Splice sites 5' cap 5' UTR (untranslated region) 3' poly A tail 3' UTR (untranslated region) Translational start (AUG) Translational stop (UGA, UAG, or UAA) N and C ends of polypeptide 0000arrow_forwardMatch the letter labels in the figure below to the terms. Some letter labels are not used. MNNNNNNIN M C B A M D F E H K G 8arrow_forwardThe diagram below illustrates a quorum sensing pathway from Staphylococcus aureus. Please answer the following questions. 1. Autoinduction is part of the quorum sensing system. Which promoter (P2 or P3) is critical for autoinduction? 2)This staphylococcus aureus grows on human wounds, causing severe infections. You would like to start a clinical trial to treat these wound infections. Please describe: a) What molecule do you recommend for the trial. Why? b) Your trial requires that Staphylococcus aureus be isolated from the wound and submitted to genome sequencing before admittance. Why? What are you testing for? 3) If a mutation arises where the Promoter P3 is constitutively active, how would that influence sensitivity to AIP? Please explain your rationale. 4) This pathway is sensitive to bacterial cell density. Describe two separate mutation that would render the pathway active independent of cell density. Briefly explain your rationale. Mutation 1 Mutation 2arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College





