
(a)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
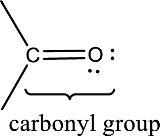
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(b)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
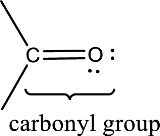
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(c)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
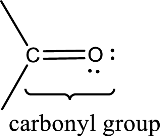
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(d)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
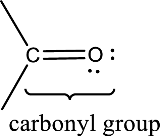
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forward
- give example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward
- 3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forwardA 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forwardThe compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





