
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 65TE
One of the skin-irritating components of poison oak is tetrahydrourushiol:
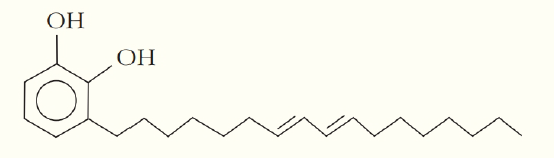
The long, nonpolar hydrocarbon tail embeds itself in a person’s oily skin, where the molecule initiates an allergic response. Scratching the itch spreads tetrahydrourushiol molecules over a greater surface area, causing the zone of irritation to grow. Is this compound an alcohol or a phenol? Defend your answer?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the Experimental Acceleration due to Gravity values from each data table, Data Tables 1, 2, and 3; determine the Standard Deviation, σ, mean, μ, variance, σ2 and the 95% Margin of Error (Confidence Level) Data: Ex. Acc. 1: 12.29 m/s^2. Ex. Acc. 2: 10.86 m/s^2, Ex. Acc. 3: 9.05 m/s^2
In the Super Smash Bros. games the character Yoshi’s has a “ground pound” down special move where he launches himself downward to attack an enemy beneath him. A) If Yoshi flings himself downwards at 9.76 miles per hour to hit an enemy 10.5 m below him, how fast is Yoshi traveling when he hits the enemy? 1 mile = 1609 m B) How much time does it take Yoshi to hit the enemy beneath him?
No chatgpt pls will upvote
Chapter 14 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
WHAT IF? What would the human life cycle be like if we had alternation of generations? Assume that the multice...
Campbell Biology (11th Edition)
Given the end results of the two types of division, why is it necessary for homologs to pair during meiosis and...
Concepts of Genetics (12th Edition)
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
Why is living epithelial tissue limited to a certain thickness?
Human Anatomy & Physiology (2nd Edition)
Modified True/False 6. __________ Halophiles inhabit extremely saline habitats, such as the Great Salt Lake.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 1.62 On a training flight, a Figure P1.62 student pilot flies from Lincoln, Nebraska, to Clarinda, Iowa, next to St. Joseph, Missouri, and then to Manhattan, Kansas (Fig. P1.62). The directions are shown relative to north: 0° is north, 90° is east, 180° is south, and 270° is west. Use the method of components to find (a) the distance she has to fly from Manhattan to get back to Lincoln, and (b) the direction (relative to north) she must fly to get there. Illustrate your solutions with a vector diagram. IOWA 147 km Lincoln 85° Clarinda 106 km 167° St. Joseph NEBRASKA Manhattan 166 km 235° S KANSAS MISSOURIarrow_forwardPlz no chatgpt pls will upvotearrow_forward3.19 • Win the Prize. In a carnival booth, you can win a stuffed gi- raffe if you toss a quarter into a small dish. The dish is on a shelf above the point where the quarter leaves your hand and is a horizontal dis- tance of 2.1 m from this point (Fig. E3.19). If you toss the coin with a velocity of 6.4 m/s at an angle of 60° above the horizontal, the coin will land in the dish. Ignore air resistance. (a) What is the height of the shelf above the point where the quarter leaves your hand? (b) What is the vertical component of the velocity of the quarter just before it lands in the dish? Figure E3.19 6.4 m/s 2.1arrow_forward
- Can someone help me answer this thank you.arrow_forward1.21 A postal employee drives a delivery truck along the route shown in Fig. E1.21. Determine the magnitude and direction of the resultant displacement by drawing a scale diagram. (See also Exercise 1.28 for a different approach.) Figure E1.21 START 2.6 km 4.0 km 3.1 km STOParrow_forwardhelp because i am so lost and it should look something like the picturearrow_forward
- 3.31 A Ferris wheel with radius Figure E3.31 14.0 m is turning about a horizontal axis through its center (Fig. E3.31). The linear speed of a passenger on the rim is constant and equal to 6.00 m/s. What are the magnitude and direction of the passenger's acceleration as she passes through (a) the lowest point in her circular motion and (b) the high- est point in her circular motion? (c) How much time does it take the Ferris wheel to make one revolution?arrow_forward1.56 ⚫. Three horizontal ropes pull on a large stone stuck in the ground, producing the vector forces A, B, and C shown in Fig. P1.56. Find the magnitude and direction of a fourth force on the stone that will make the vector sum of the four forces zero. Figure P1.56 B(80.0 N) 30.0 A (100.0 N) 53.0° C (40.0 N) 30.0°arrow_forward1.39 Given two vectors A = -2.00 +3.00 +4.00 and B=3.00 +1.00 -3.00k. (a) find the magnitude of each vector; (b) use unit vectors to write an expression for the vector difference A - B; and (c) find the magnitude of the vector difference A - B. Is this the same as the magnitude of B - Ä? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning



Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Stars and Galaxies
Physics
ISBN:9781305120785
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY