
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 89TE
The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A convex mirror (f.=-6.20cm) and a concave minor (f2=8.10 cm)
distance of 15.5cm
are facing each other and are separated by a
An object is placed between the mirrors and is 7.8cm from each
mirror. Consider the light from the object that reflects first from
the convex mirror and then from the concave mirror. What is the
distance of the image (dia) produced by the concave mirror?
cm.
An amusement park spherical mirror shows
park spherical mirror shows anyone who stands
2.80m in front of it an upright image
one
and a half times the
person's height. What is the focal length of the minor?
m.
An m = 69.0-kg person running at an initial speed of v = 4.50 m/s jumps onto an M = 138-kg cart initially at rest (figure below). The person slides on the cart's top surface and finally comes to rest relative to the cart. The coefficient of kinetic friction between the person and the cart is
0.440. Friction between the cart and ground can be ignored. (Let the positive direction be to the right.)
m
M
(a) Find the final velocity of the person and cart relative to the ground. (Indicate the direction with the sign of your answer.)
m/s
(b) Find the friction force acting on the person while he is sliding across the top surface of the cart. (Indicate the direction with the sign of your answer.)
N
(c) How long does the friction force act on the person?
S
(d) Find the change in momentum of the person. (Indicate the direction with the sign of your answer.)
N.S
Find the change in momentum of the cart. (Indicate the direction with the sign of your answer.)
N.S
(e) Determine the displacement of the…
Chapter 14 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
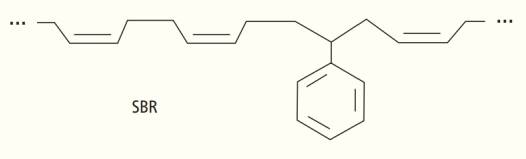
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
33. Consider the reaction:
The tabulated data were collected for the concentration of C4H8 as a function...
Chemistry: Structure and Properties (2nd Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Use the key to classify each of the following described tissue types into one of the four major tissue categori...
Anatomy & Physiology (6th Edition)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
Why is an endospore called a resting structure? Of what advantage is an endospore to a bacterial cell?
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Small ice cubes, each of mass 5.60 g, slide down a frictionless track in a steady stream, as shown in the figure below. Starting from rest, each cube moves down through a net vertical distance of h = 1.50 m and leaves the bottom end of the track at an angle of 40.0° above the horizontal. At the highest point of its subsequent trajectory, the cube strikes a vertical wall and rebounds with half the speed it had upon impact. If 10 cubes strike the wall per second, what average force is exerted upon the wall? N ---direction--- ▾ ---direction--- to the top to the bottom to the left to the right 1.50 m 40.0°arrow_forwardThe magnitude of the net force exerted in the x direction on a 3.00-kg particle varies in time as shown in the figure below. F(N) 4 3 A 2 t(s) 1 2 3 45 (a) Find the impulse of the force over the 5.00-s time interval. == N⚫s (b) Find the final velocity the particle attains if it is originally at rest. m/s (c) Find its final velocity if its original velocity is -3.50 î m/s. V₁ m/s (d) Find the average force exerted on the particle for the time interval between 0 and 5.00 s. = avg Narrow_forward••63 SSM www In the circuit of Fig. 27-65, 8 = 1.2 kV, C = 6.5 µF, R₁ S R₂ R3 800 C H R₁ = R₂ = R3 = 0.73 MQ. With C completely uncharged, switch S is suddenly closed (at t = 0). At t = 0, what are (a) current i̟ in resistor 1, (b) current 2 in resistor 2, and (c) current i3 in resistor 3? At t = ∞o (that is, after many time constants), what are (d) i₁, (e) i₂, and (f) iz? What is the potential difference V2 across resistor 2 at (g) t = 0 and (h) t = ∞o? (i) Sketch V2 versus t between these two extreme times. Figure 27-65 Problem 63.arrow_forward
- Thor flies by spinning his hammer really fast from a leather strap at the end of the handle, letting go, then grabbing it and having it pull him. If Thor wants to reach escape velocity (velocity needed to leave Earth’s atmosphere), he will need the linear velocity of the center of mass of the hammer to be 11,200 m/s. Thor's escape velocity is 33532.9 rad/s, the angular velocity is 8055.5 rad/s^2. While the hammer is spinning at its maximum speed what impossibly large tension does the leather strap, which the hammer is spinning by, exert when the hammer is at its lowest point? the hammer has a total mass of 20.0kg.arrow_forwardIf the room’s radius is 16.2 m, at what minimum linear speed does Quicksilver need to run to stay on the walls without sliding down? Assume the coefficient of friction between Quicksilver and the wall is 0.236.arrow_forwardIn the comics Thor flies by spinning his hammer really fast from a leather strap at the end of the handle, letting go, then grabbing it and having it pull him. If Thor wants to reach escape velocity (velocity needed to leave Earth’s atmosphere), he will need the linear velocity of the center of mass of the hammer to be 11,200 m/s. A) If the distance from the end of the strap to the center of the hammer is 0.334 m, what angular velocity does Thor need to spin his hammer at to reach escape velocity? b) If the hammer starts from rest what angular acceleration does Thor need to reach that angular velocity in 4.16 s? c) While the hammer is spinning at its maximum speed what impossibly large tension does the leather strap, which the hammer is spinning by, exert when the hammer is at its lowest point? The hammer has a total mass of 20.0kg.arrow_forward
- The car goes from driving straight to spinning at 10.6 rev/min in 0.257 s with a radius of 12.2 m. The angular accleration is 4.28 rad/s^2. During this flip Barbie stays firmly seated in the car’s seat. Barbie has a mass of 58.0 kg, what is her normal force at the top of the loop?arrow_forwardConsider a hoop of radius R and mass M rolling without slipping. Which form of kinetic energy is larger, translational or rotational?arrow_forwardA roller-coaster vehicle has a mass of 571 kg when fully loaded with passengers (see figure). A) If the vehicle has a speed of 22.5 m/s at point A, what is the force of the track on the vehicle at this point? B) What is the maximum speed the vehicle can have at point B, in order for gravity to hold it on the track?arrow_forward
- This one wheeled motorcycle’s wheel maximum angular velocity was about 430 rev/min. Given that it’s radius was 0.920 m, what was the largest linear velocity of the monowheel?The monowheel could not accelerate fast or the rider would start spinning inside (this is called "gerbiling"). The maximum angular acceleration was 10.9 rad/s2. How long, in seconds, would it take it to hit maximum speed from rest?arrow_forwardIf points a and b are connected by a wire with negligible resistance, find the magnitude of the current in the 12.0 V battery.arrow_forwardConsider the two pucks shown in the figure. As they move towards each other, the momentum of each puck is equal in magnitude and opposite in direction. Given that v kinetic energy of the system is converted to internal energy? 30.0° 130.0 = green 11.0 m/s, and m blue is 25.0% greater than m 'green' what are the final speeds of each puck (in m/s), if 1½-½ t thearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY