
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 91TE
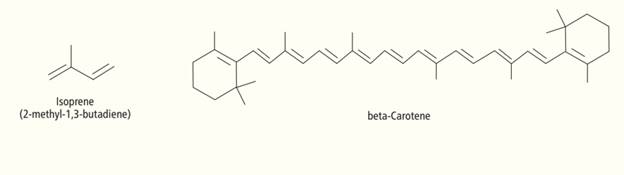
Many of the natural product molecules synthesized by photosynthetic plants are formed by the
joining together of isoprene monomers via an addition
nutrient beta-carotene. How many isoprene units are needed to make one beta-carotene
molecule? (Hint: Count the carbons in each.) Find and circle these units within the beta-carotene
structure shown here.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Make sure to draw a Free Body Diagram as well
Make sure to draw a Free Body Diagram as well
Make sure to draw a Free Body Diagram please as well
Chapter 14 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
Given the end results of the two types of division, why is it necessary for homologs to pair during meiosis and...
Concepts of Genetics (12th Edition)
Assume that genes, A and B are on the same chromosome and are 50 map units apart. An animal heterozygous at bot...
Campbell Biology (11th Edition)
Which culture uses NAD+? Use the following choices to answer questions. a. E. coli growing in glucose broth at ...
Microbiology: An Introduction
13. The peak current through a capacitor is 2.0 A. What is the peak current if
a. The peak emf ?0 is doubled?
b...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- please answer this asap!!!!arrow_forwardRT = 4.7E-30 18V IT = 2.3E-3A+ 12 38Ω ли 56Ω ли r5 27Ω ли r3 28Ω r4 > 75Ω r6 600 0.343V 75.8A Now figure out how much current in going through the r4 resistor. |4 = unit And then use that current to find the voltage drop across the r resistor. V4 = unitarrow_forward7 Find the volume inside the cone z² = x²+y², above the (x, y) plane, and between the spheres x²+y²+z² = 1 and x² + y²+z² = 4. Hint: use spherical polar coordinates.arrow_forward
- ганм Two long, straight wires are oriented perpendicular to the page, as shown in the figure(Figure 1). The current in one wire is I₁ = 3.0 A, pointing into the page, and the current in the other wire is 12 4.0 A, pointing out of the page. = Find the magnitude and direction of the net magnetic field at point P. Express your answer using two significant figures. VO ΜΕ ΑΣΦ ? Figure P 5.0 cm 5.0 cm ₁ = 3.0 A 12 = 4.0 A B: μΤ You have already submitted this answer. Enter a new answer. No credit lost. Try again. Submit Previous Answers Request Answer 1 of 1 Part B X Express your answer using two significant figures. ΜΕ ΑΣΦ 0 = 0 ? below the dashed line to the right P You have already submitted this answer. Enter a new answer. No credit lost. Try again.arrow_forwardAn infinitely long conducting cylindrical rod with a positive charge λ per unit length is surrounded by a conducting cylindrical shell (which is also infinitely long) with a charge per unit length of −2λ and radius r1, as shown in the figure. What is σinner, the surface charge density (charge per unit area) on the inner surface of the conducting shell? What is σouter, the surface charge density on the outside of the conducting shell? (Recall from the problem statement that the conducting shell has a total charge per unit length given by −2λ.)arrow_forwardA small conducting spherical shell with inner radius aa and outer radius b is concentric with a larger conducting spherical shell with inner radius c and outer radius d (Figure 1). The inner shell has total charge +2q, and the outer shell has charge −2q. What's the total charge on the inner surface of the small shell? What's the total charge on the outer surface of the small shell? What's the total charge on the inner surface of the large shell? What's the total charge on the outer surface of the large shell?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY