
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 80TE
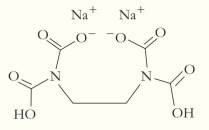
The disodium salt of ethylenediaminetetraacetic acid, also known as
for lead ions,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6. A car drives at steady speed around a perfectly
circular track.
(a) The car's acceleration is zero.
(b) The net force on the car is zero.
(c) Both the acceleration and net force on the car
point outward.
(d) Both the acceleration and net force on the car
point inward.
(e) If there is no friction, the acceleration is
outward.
9. A spring has a force constant of 100 N/m and an
unstretched length of 0.07 m. One end is attached to
a post that is free to rotate in the center of a smooth.
table, as shown in the top view in the figure below.
The other end is attached to a 1kg disc moving in
uniform circular motion on the table, which
stretches the spring by 0.03 m. Friction is negligible.
What is the centripetal force on the disc?
Top View
(a)
0.3 N
(b)
3.0 N
(c)
10 N
(d)
300 N
(e)
1000 N
4. A child has a ball on the end of a cord, and whirls
the ball in a vertical circle. Assuming the speed of
the ball is constant (an approximation), when would
the tension in the cord be greatest?
(a) At the top of the circle.
(b) At the bottom of the circle.
(c) A little after the bottom of the circle when the
ball is climbing.
(d) A little before the bottom of the circle when the
ball is descending quickly.
(e) Nowhere; the cord is pulled the same amount at
all points.
Chapter 14 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
What is the reducing agent in the following reaction?
2 Br –– (aq) + H2 O2 (aq) + 2 H+ (aq) → Br2 (aq) + 2 H2 ...
Chemistry: The Central Science (14th Edition)
EVOLUTION CONNECTION The percentages of naturally occurring elements making up the human body (see Table 2.1) a...
Campbell Biology (11th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Based on your answers to Questions 2 and 3, which part of the Atlantic basin appears to have opened first?
Applications and Investigations in Earth Science (9th Edition)
What are four functions of connective tissue?
Anatomy & Physiology (6th Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3. In a rotating vertical cylinder (Rotor-ride) a rider finds herself pressed with her back to the rotating wall. Which is the correct free-body diagram for her? (a) (b) (c) (d) (e)arrow_forward8. A roller coaster rounds the bottom of a circular loop at a nearly constant speed. At this point the net force on the coaster cart is (a) zero. (b) directed upward. (c) directed downward. (d) Cannot tell without knowing the exact speed.arrow_forward5. While driving fast around a sharp right turn, you find yourself pressing against the left car door. What is happening? (a) Centrifugal force is pushing you into the door. (b) The door is exerting a rightward force on you. (c) Both of the above. (d) Neither of the above.arrow_forward
- 7. You are flung sideways when your car travels around a sharp curve because (a) you tend to continue moving in a straight line. (b) there is a centrifugal force acting on you. (c) the car exerts an outward force on you. (d) of gravity.arrow_forward1. A 50-N crate sits on a horizontal floor where the coefficient of static friction between the crate and the floor is 0.50. A 20-N force is applied to the crate acting to the right. What is the resulting static friction force acting on the crate? (a) 20 N to the right. (b) 20 N to the left. (c) 25 N to the right. (d) 25 N to the left. (e) None of the above; the crate starts to move.arrow_forward3. The problem that shall not be named. m A (a) A block of mass m = 1 kg, sits on an incline that has an angle 0. Find the coefficient of static friction by analyzing the system at imminent motion. (hint: static friction will equal the maximum value) (b) A block of mass m = 1kg made of a different material, slides down an incline that has an angle 0 = 45 degrees. If the coefficient of kinetic friction increases is μ = 0.5 what is the acceleration of the block? karrow_forward
- 2. Which of the following point towards the center of the circle in uniform circular motion? (a) Acceleration. (b) Velocity, acceleration, net force. (c) Velocity, acceleration. (d) Velocity, net force. (e) Acceleration, net force.arrow_forwardProblem 1. (20 pts) The third and fourth stages of a rocket are coastin in space with a velocity of 18 000 km/h when a smal explosive charge between the stages separate them. Immediately after separation the fourth stag has increased its velocity to v4 = 18 060 km/h. Wha is the corresponding velocity v3 of the third stage At separation the third and fourth stages hav masses of 400 and 200 kg, respectively. 3rd stage 4th stagearrow_forwardMany experts giving wrong answer of this question. please attempt when you 100% sure . Otherwise i will give unhelpful.arrow_forward
- Determine the shear and moment diagram for the beam shown in Fig.1. A 2 N/m 10 N 8 N 6 m B 4m Fig.1 40 Nm Steps: 1) Determine the reactions at the fixed support (RA and MA) (illustrated in Fig 1.1) 2) Draw the free body diagram on the first imaginary cut (fig. 1.2), and determine V and M. 3) Draw the free body diagram on the second imaginary cut (fig. 1.3), and determine V and M. 4) Draw the shear and moment diagramarrow_forwardConsidering the cross-sectional area shown in Fig.2: 1. Determine the coordinate y of the centroid G (0, ỹ). 2. Determine the moment of inertia (I). 3. Determine the moment of inertia (Ir) (with r passing through G and r//x (// parallel). 4 cm 28 cm G3+ G 4 cm y 12 cm 4 cm 24 cm xarrow_forwardI need help understanding 7.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY