
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 31P
Assign structures to each of the compounds A, B, and C whose
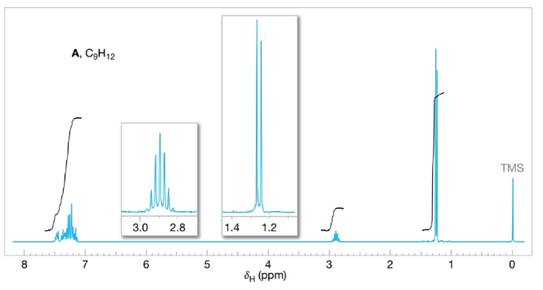
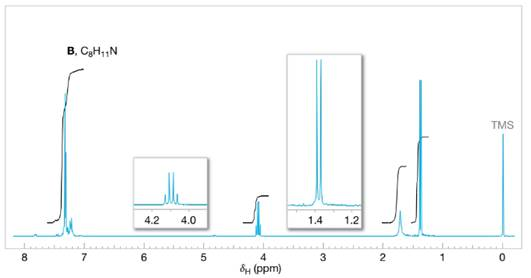
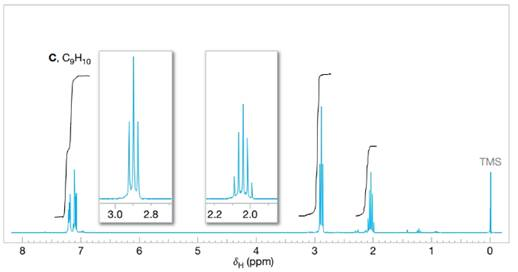
FIGURE 14.27 The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
O
? A
. If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
. If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
ㅇ
80
F5
F6
A
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente
FIG
In methyl orange preparation, if the reaction started with 0.5 mole of sulfanilic acid to form the diazonium salt of this compound and then it converted to methyl orange [0.2 mole]. If the efficiency of the second step was 50%, Calculate: A. Equation(s) of Methyl Orange synthesis: Diazotization and coupling reactions. B. How much diazonium salt was formed in this reaction? C. The efficiency percentage of the diazotization reaction D. Efficiency percentage of the whole reaction.
Hand written equations please
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
If a compound has a molecular ion with an odd-numbered mass, then the compound contains an odd number of nitrog...
Organic Chemistry (8th Edition)
What is the importance of granulation tissue?
Principles of Anatomy and Physiology
15. A good scientific hypothesis is based on existing evidence and leads to testable predictions. What hypothes...
Campbell Biology: Concepts & Connections (9th Edition)
The number of named species is about ________, but the actual number of species on Earth is estimated to be abo...
Biology: Life on Earth with Physiology (11th Edition)
Which element is a maingroup metal with an even atomic number? a. K b. Ca c. Cr d. Se
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forwardNMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forward
- How many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forward
- in the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forward
- I2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY