
Concept explainers
(a) In 1960 T. Katz (Columbia University) showed that cyclooctatetraene adds two electrons when treated with potassium metal and forms a stable, planar dianion,
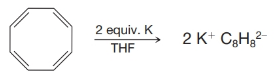
Use the molecular orbital diagram given in Fig. 14.7 and explain this result.
(b) In 1964 Katz also showed that removing two protons from the compound below (using butyllithium as the base) leads to the forma-tion of a stable dianion with the formula

Propose a reasonable structure for the product and explain why it is stable.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Fundamentals Of Thermodynamics
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Microbiology with Diseases by Body System (5th Edition)
Living By Chemistry: First Edition Textbook
- Complete the following equations hand written pleasearrow_forwardComplete the following equations please hand written pleasearrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forward
- Please help me solve this homework problemarrow_forwardPlease help me answer this homework questionarrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





