
(a)
Interpretation:
The following
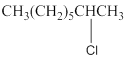
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms.
The compounds in which hydrogen atoms of an
When the halogen is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the halogen is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the halogen is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
(b)
Interpretation:
The following alkyl halide should be classified as
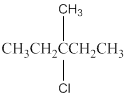
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The compounds in which hydrogen atoms of an alkane is replaced by halogen is known as alkyl halide.
When the halogen is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the halogen is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the halogen is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
(c)
Interpretation:
The following alkyl halide should be classified as
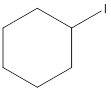
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The compounds in which hydrogen atoms of an alkane is replaced by halogen is known as alkyl halide.
When the halogen is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the halogen is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the halogen is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
(d)
Interpretation:
The following alkyl halide should be classified as
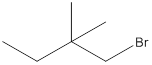
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The compounds in which hydrogen atoms of an alkane is replaced by halogen is known as alkyl halide.
When the halogen is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the halogen is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the halogen is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Don't used Ai solutionarrow_forwardLet's see if you caught the essentials of the animation. What is the valence value of carbon? a) 4 b) 2 c) 8 d) 6arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forward
- A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardThe number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forwardFor the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





