
Concept explainers
(a)
Interpretation:
Structure of the 3-hexanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as
Answer to Problem 39P
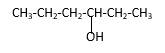
Explanation of Solution
Structure of the 3-hexanol contains six carbon length main carbon chain which connects to an alcohol group in the 3rd carbon of the main carbon chain. There are no sub groups which connect to it. And according to the structure it should be a secondary alcohol.
According to the name, structure of the compound is as below;
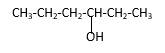
(b)
Interpretation:
Structure of the propyl alcohol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
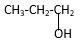
Explanation of Solution
Structure of the propyl alcohol consist one main C chain which contains three C atoms, and alcohol group is connected to the 3rdposition of the main C chain. As per the name it should be a primary alcohol.
Structure of the compound is as below;
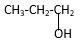
(c)
Interpretation:
Structure of the 2-methylcyclopropanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
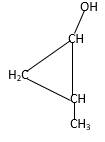
Explanation of Solution
Structure of the 2-methylcyclopropanol consist one main C ring which contains three C atoms, and alcohol group is connected to the 1stposition of the main C ring. Methyl groupis connected to the2nd position of the main C ring. And as per the name it should be a primary alcohol.
Structure of the compound is as below;
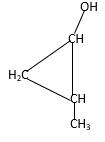
(d)
Interpretation:
Structure of the 1,2-butanediol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
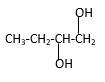
Explanation of Solution
Structure of the 1,2-butanediol consist one main C chain which contains four C atoms, and alcohol groupsare connected to the 2ndand 1stpositions of the main C chain.
Structure of the compound is as below;

(e)
Interpretation:
Structure of the 4,4,5-trimethyl-3-heptanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
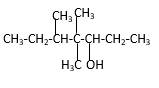
Explanation of Solution
Structure of the 4,4,5-trimethyl-3-heptanol consist one main C chain which contains seven C atoms, and alcohol group is connected to the 3rdposition of the main C chain. Out of three methyl groups, twoare connects to the 4th position of the main C chain and one is connected to the 5th position of the main carbon chain. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;

(f)
Interpretation:
Structure of the 3,5-dimethyl-1-heptanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 39P
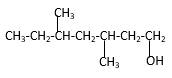
Explanation of Solution
Structure of the 3,5-dimethyl-1-heptanol consist one main C chain which contains seven C atoms, and alcohol group is connected to the 1st position of the main C chain. Methyl groupsare connected to the 3rd and 5th positions of the main C chain. And as per the name it should be a primary alcohol.
Structure of the compound is as below;

Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, and Biological Chemistry - 4th edition
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





