
Concept explainers
To review:
The structures ofproducts of the reaction between nitrogenase complex and substrates hydrogen cyanide, dinitroge, nnd acetylene.
Introduction:
Nitrogenase complex is present in all the species that can fix nitrogen. It is produced by bacteria such as cyanobacteria. This reduces nitrogen (N2) to ammonia (NH3). This is the only enzyme that can catalyze the reaction for nitrogen fixation.
Explanation of Solution
The nitrogenase complex consists of two proteins called dinitrogenase as well asdinitrogenase reductase. The dinitrogenase is heterotetramer of MoFe protein. The dinitrogenase reductase is called Fe(ferrous) protein. It contains Mg-ATP (magnesium-adenosine triphosphate)-binding sites. In the reaction catalyzed by this complex, there is a transfer of electrons from (4Fe-4S) cluster in Fe protein. The Fe transfers the electrons to Mo-Fe protein. The transfer of electrons leads to a reduction of H+. The incoming N2 exchanges withH+ within the active site to form stable intermediates. So, the reaction of nitrogenasecomplex with the given substrates is as follows:
When nitrogenase complex reacts with cyanide, it gives two product, sethylamine and methan, elong with ammonia:
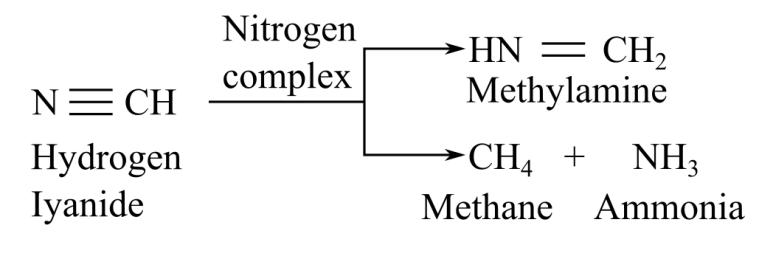
When nitrogenase complex reacts with acetylene, it gives ethylene as a product:
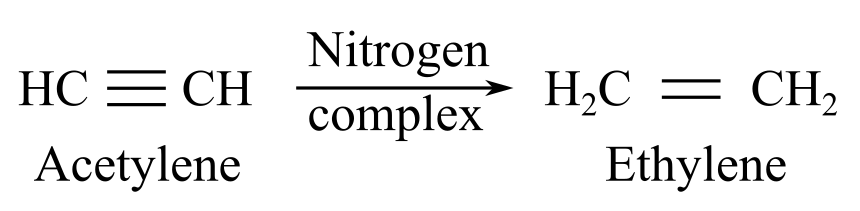
When nitrogenase complex reacts with dinitrogen, it gives hydrazine or ammonia as a product:
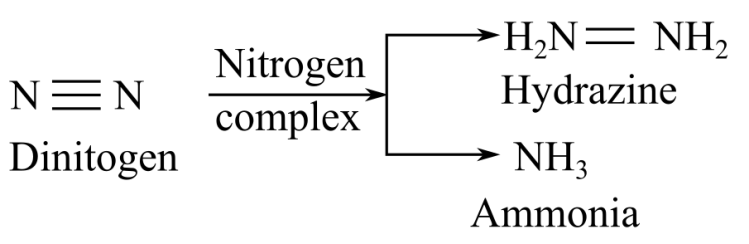
Thus, it can be concluded that the products of substrates hydrogen cyanide, acetylen, endnitrogen are methylamine, ethylene, and hydrazine, respectively.
Want to see more full solutions like this?
Chapter 14 Solutions
Biochemistry: The Molecular Basis of Life
- Do sensory neurons express ACE2 or only neurolipin-1 receptors for COVID19 virus particle binding?arrow_forwardExplain the process of CNS infiltration of COVID19 through sensory neurons from beginning to end, including processes like endocytosis, the different receptors/proteins that are involved, how they are transported and released, etc.,arrow_forwardH2C CH2 HC-COOO CH2 ܘHO-C-13c-O isocitrate C-S-COA H213c CH2 C-OO 13C-S-COA CH2 C-00 the label will not be present in succinyl CoA C-S-COA succinyl-CoAarrow_forward
- A culture of kidneys cells contains all intermediates of the citric acid cycle. It is treated with an irreversible inhibitor of malate dehydrogenase, and then infused withglucose. Fill in the following list to account for the number of energy molecules that are formed from that one molecule of glucose in this situation. (NTP = nucleotidetriphosphate, e.g., ATP or GTP)Net number of NTP:Net number of NADH:Net number of FADH2:arrow_forward16. Which one of the compounds below is the final product of the reaction sequence shown here? OH A B NaOH Zn/Hg aldol condensation heat aq. HCI acetone C 0 D Earrow_forward2. Which one of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H₂/Pd? A B C D Earrow_forward
- 6. What is the IUPAC name of the following compound? A) (Z)-3,5,6-trimethyl-3,5-heptadiene B) (E)-2,3,5-trimethyl-1,4-heptadiene C) (E)-5-ethyl-2,3-dimethyl-1,5-hexadiene D) (Z)-5-ethyl-2,3-dimethyl-1,5-hexadiene E) (Z)-2,3,5-trimethyl-1,4-heptadienearrow_forwardConsider the reaction shown. CH2OH Ex. CH2 -OH CH2- Dihydroxyacetone phosphate glyceraldehyde 3-phosphate The standard free-energy change (AG) for this reaction is 7.53 kJ mol-¹. Calculate the free-energy change (AG) for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde 3-phosphate] = 0.00300 M. AG= kJ mol-1arrow_forwardIf the pH of gastric juice is 1.6, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the membrane potential across this membrane is -70.0 mV and the temperature is 37 °C. AG= kJ mol-1arrow_forward
- Consider the fatty acid structure shown. Which of the designations are accurate for this fatty acid? 17:2 (48.11) 18:2(A9.12) cis, cis-A8, A¹¹-octadecadienoate w-6 fatty acid 18:2(A6,9)arrow_forwardClassify the monosaccharides. H-C-OH H. H-C-OH H-C-OH CH₂OH H-C-OH H-C-OH H-C-OH CH₂OH CH₂OH CH₂OH CH₂OH D-erythrose D-ribose D-glyceraldehyde Dihydroxyacetone CH₂OH CH₂OH C=O Answer Bank CH₂OH C=0 HO C-H C=O H-C-OH H-C-OH pentose hexose tetrose H-C-OH H-C-OH H-C-OH aldose triose ketose CH₂OH CH₂OH CH₂OH D-erythrulose D-ribulose D-fructosearrow_forwardFatty acids are carboxylic acids with long hydrophobic tails. Draw the line-bond structure of cis-A9-hexadecenoate. Clearly show the cis-trans stereochemistry.arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





