
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14, Problem 14.74CP
a.
Interpretation Introduction
Interpretation:
Acylic monosaccharide has to be drawn for the given cyclic form.
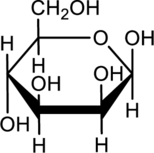
b.
Interpretation Introduction
Interpretation:
Acylic monosaccharide has to be drawn for the given cyclic form.
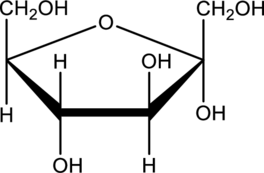
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Given the first-order reaction: aA → products. State its kinetic equation.
Determine the symmetry of the
combination of atomic orbitals for bf
4-
H
Draw the product and the mechanism
1.) LDA
+
2.) H30*
H
○ excess
OH
Chapter 14 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 14.1 - Draw a Lewis structure for glucose that clearly...Ch. 14.2 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Rank the following compounds in order of...Ch. 14.2 - Prob. 14.6PCh. 14.2 - Prob. 14.7PCh. 14.2 - Prob. 14.8PCh. 14.2 - Prob. 14.9PCh. 14.3 - Prob. 14.10P
Ch. 14.3 - Prob. 14.11PCh. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13PCh. 14.4 - Prob. 14.14PCh. 14.4 - Prob. 14.15PCh. 14.5 - Prob. 14.16PCh. 14.5 - Prob. 14.17PCh. 14.5 - Prob. 14.18PCh. 14.5 - Prob. 14.19PCh. 14.6 - Prob. 14.20PCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - Prob. 14.26UKCCh. 14 - Prob. 14.27UKCCh. 14 - Prob. 14.28UKCCh. 14 - Prob. 14.29UKCCh. 14 - Prob. 14.30UKCCh. 14 - Prob. 14.31APCh. 14 - Prob. 14.32APCh. 14 - Prob. 14.33APCh. 14 - Prob. 14.34APCh. 14 - Prob. 14.35APCh. 14 - Prob. 14.36APCh. 14 - Prob. 14.37APCh. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Prob. 14.44APCh. 14 - Prob. 14.45APCh. 14 - Prob. 14.46APCh. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - Prob. 14.49APCh. 14 - Prob. 14.50APCh. 14 - Prob. 14.51APCh. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Prob. 14.58APCh. 14 - Prob. 14.59APCh. 14 - Prob. 14.60APCh. 14 - Prob. 14.61APCh. 14 - Prob. 14.62APCh. 14 - Prob. 14.63APCh. 14 - Prob. 14.64APCh. 14 - Prob. 14.65APCh. 14 - Prob. 14.66APCh. 14 - Prob. 14.67APCh. 14 - Prob. 14.68APCh. 14 - Prob. 14.69APCh. 14 - Prob. 14.70APCh. 14 - Prob. 14.71APCh. 14 - Prob. 14.72APCh. 14 - Prob. 14.73CPCh. 14 - Prob. 14.74CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning