
Concept explainers
a.
Interpretation:
Fischer projection formula has to be drawn for the ball-and-stick model of the monosaccharide given and also it has to be labelled as D or L sugar.
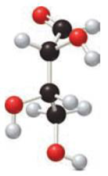
Concept Introduction:
Fischer projection formula simply uses cross for representing tetrahedral carbon atom. The carbon atom that is present in the intersection point in cross. The horizontal bonds means they are coming forward and they are present on wedge bond. The vertical bonds means they are pointing away and they are present on dashed lines.
A carbon atom that is bonded to four different groups is known as a chiral carbon atom. This can rotate the plane polarized light. D- and L- isomers of monosaccharide can be identified by looking into the chiral center that is farther from the carbonyl group. In a Fischer projection, if the
b.
Interpretation:
Given monosaccharide has to be classified based on the number of carbon atoms present in the chain and also the carbonyl group.
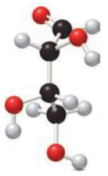
Concept Introduction:
Simplest carbohydrates are known as monosaccharides. They contain three to six carbons generally in a chain form with a carbonyl group present in the terminal or the adjacent carbon atom from the terminal. Monosaccharides that have the carbonyl group at the terminal carbon atom
The number of carbon atoms present in the chain characterize the monosaccharide. They are given below.
- Carbon chain with three carbon atoms is triose.
- Carbon chain with four carbon atoms is tetrose.
- Carbon chain with five carbon atoms is as pentose.
- Carbon chain with six carbon atoms is as hexose.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
PRIN.OF GENERAL,ORGANIC+BIOLOG.CHEM.
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





