
Concept explainers
a.
Interpretation:
Given monosaccharides has to be classified based on the number of carbon atoms present in the chain and also the carbonyl group.
Concept Introduction:
Simplest carbohydrates are known as monosaccharides. They contain three to six carbons generally in a chain form with a carbonyl group present in the terminal or the adjacent carbon atom from the terminal. Monosaccharides that have the carbonyl group at the terminal carbon atom
The number of carbon atoms present in the chain characterize the monosaccharide. They are given below.
- Carbon chain with three carbon atoms is triose.
- Carbon chain with four carbon atoms is tetrose.
- Carbon chain with five carbon atoms is as pentose.
- Carbon chain with six carbon atoms is as hexose.
a.
Explanation of Solution
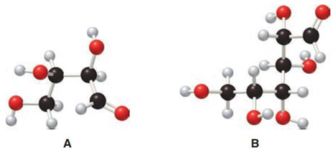
Structure of monosaccharide A is drawn as shown below.
The carbonyl group is present on the terminal carbon atom
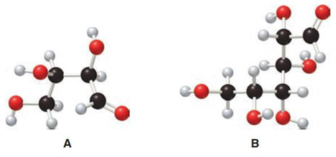
Structure of monosaccharide B is drawn as shown below.
The carbonyl group is present on the terminal carbon atom
b.
Interpretation:
Chiral centers has to be located in the given compounds.
b.
Explanation of Solution
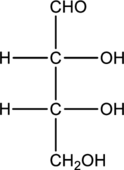
Structure of monosaccharide A is drawn as shown below.
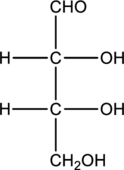
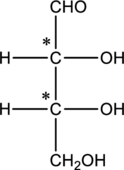
The two carbon atoms that are present in the middle is found to be bonded with four different groups. Thus, there are two chiral centers present in monosaccharide A. This is depicted as follows.
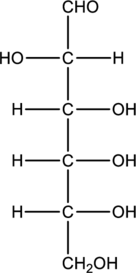
Structure of monosaccharide B is drawn as shown below.
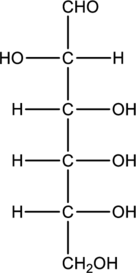
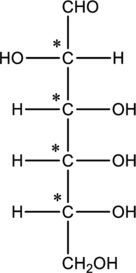
The four carbon atoms that are present in the middle is found to be bonded with four different groups. Thus, there are four chiral centers present in monosaccharide B. This is depicted as follows.
Want to see more full solutions like this?
Chapter 14 Solutions
PRIN.OF GENERAL,ORGANIC+BIOLOG.CHEM.
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





