
Assign an IUPAC name to each of the following compounds.
- a. CH3—(CH2)4—SH
- b. CH3—(CH2)4—OH
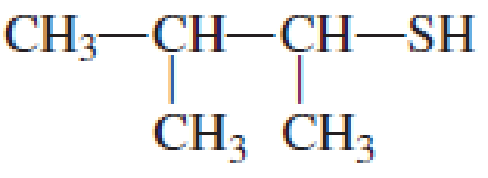
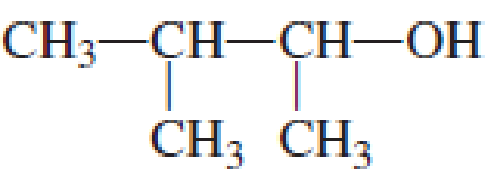
(a)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming thioalcohols that contain single thiol group:
- • Longest carbon chain has to be identified that contains thiol group also. The chain name is obtained by adding the suffix “-thiol”. If the compound contains an unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the thiol group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the thiol group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
IUPAC rules for naming thioalcohols that contain more than one thiol group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of thiol groups that is present.
Answer to Problem 14.139EP
IUPAC name for the given compound is 1-pentanethiol.
Explanation of Solution
Given structure of compound is
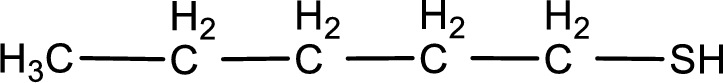
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is five carbon chain. Hence, the parent alkane is pentane.
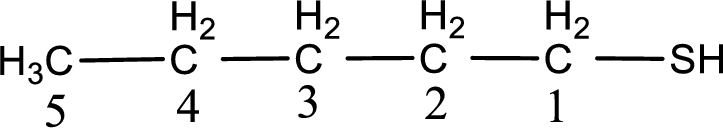
The suffix -thiol is added to the parent alkane name. This gives the name as pentanethiol.
Next step is to number the carbon atoms so that the thiol functional group gets the least numbering. In this case, it is in first carbon atom. Therefore, the IUPAC name of the given compound is 1-pentanethiol.
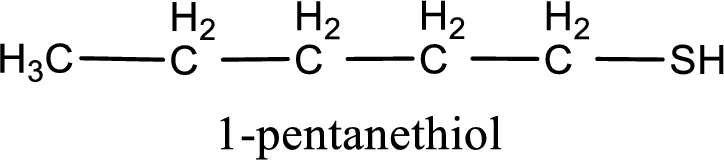
IUPAC name for the given compound is assigned.
(b)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming alcohols that contain single hydroxyl group:
- • Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”. If the compound contains a unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the hydroxyl group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- • Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
- • If the compound contains bulky groups on same side of the double bond, then it is a cis isomer and if the bulkyl groups are present on opposite side of the double bond, then it is a trans isomer.
- • In case of cycloalkane compounds, if the substitutions are present on same side of the ring of carbon atoms, it is a cis isomer. If the substitutions are present above and below the ring, then it is a trans isomer.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.139EP
IUPAC name for the given compound is 1-pentanol.
Explanation of Solution
Given structure of compound is
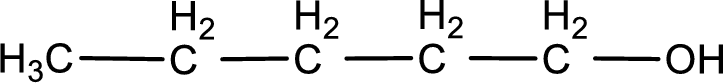
The longest continuous carbon chain with the hydroxyl group is found to be five carbon chain. Therefore, the parent alkane is pentane. As a hydroxyl group is present the name of the alcohol can be given as pentanol.
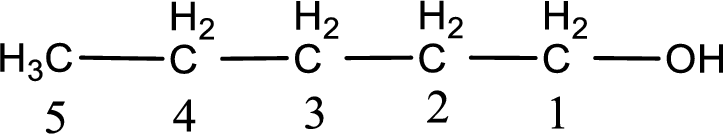
The numbering has to be given so that the hydroxyl group gets the least numbering. In this case, the hydroxyl group is present in the first carbon atom. Therefore, the IUPAC name can be given as 1-pentanol.
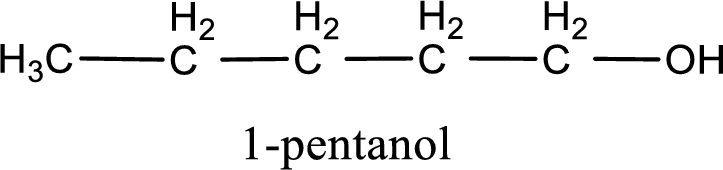
IUPAC name for the given compound is assigned.
(c)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming thioalcohols that contain single thiol group:
- • Longest carbon chain has to be identified that contains thiol group also. The chain name is obtained by adding the suffix “-thiol”. If the compound contains an unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the thiol group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the thiol group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
IUPAC rules for naming thioalcohols that contain more than one thiol group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of thiol groups that is present.
Answer to Problem 14.139EP
IUPAC name for the given compound is 3-methyl-2-butanethiol.
Explanation of Solution
Given structure of compound is shown below,
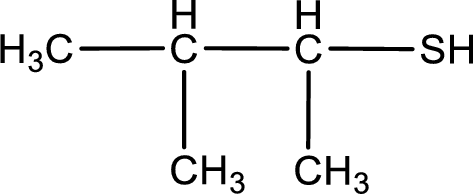
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is four carbon chain. Hence, the parent alkane is butane.
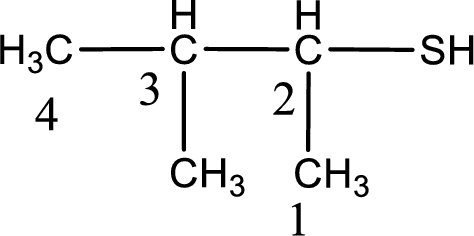
The suffix -thiol is added to the parent alkane name. This gives the name as butanethiol.
Next step is to number the carbon atoms so that the thiol functional group gets the least numbering. In this case, it is in second carbon atom. Methyl group is present as substituent in the third carbon atom. Therefore, the IUPAC name of the given compound is 3-methyl-2-butanethiol.
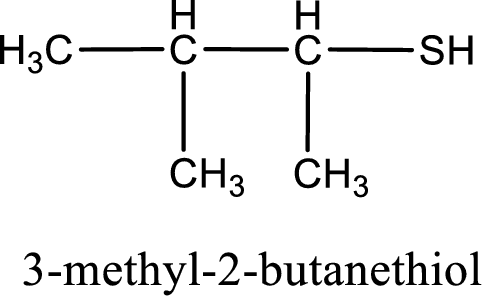
IUPAC name for the given compound is assigned.
(d)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming alcohols that contain single hydroxyl group:
- • Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”. If the compound contains a unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the hydroxyl group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- • Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
- • If the compound contains bulky groups on same side of the double bond, then it is a cis isomer and if the bulkyl groups are present on opposite side of the double bond, then it is a trans isomer.
- • In case of cycloalkane compounds, if the substitutions are present on same side of the ring of carbon atoms, it is a cis isomer. If the substitutions are present above and below the ring, then it is a trans isomer.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.139EP
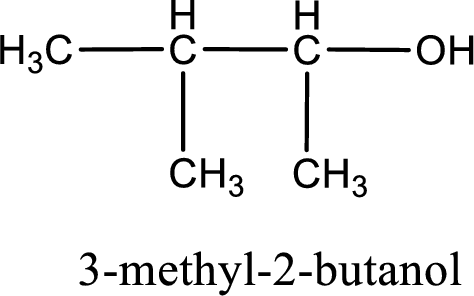
IUPAC name for the given compound is 3-methyl-2-butanol.
Explanation of Solution
Given structure of compound is shown below,
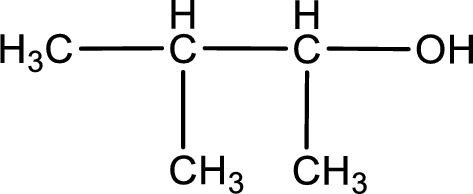
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is four carbon chain. Hence, the parent alkane is butane.
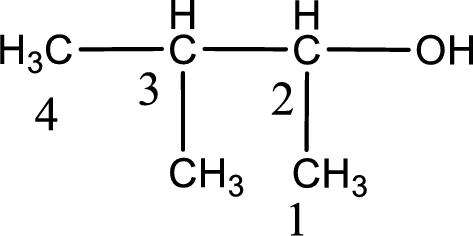
The suffix -ol is added to the parent alkane name by replacing the suffix “–e”. This gives the name as butanol.
Next step is to number the carbon atoms so that the hydroxyl functional group gets the least numbering. In this case, it is in second carbon atom. Methyl group is present as substituent in the third carbon atom. Therefore, the IUPAC name of the given compound is 3-methyl-2-butanol.
IUPAC name for the given compound is assigned.
Want to see more full solutions like this?
Chapter 14 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



