
Each of the following names is wrong. Give the structure and correct name of
each compound.
a
b
c.
d.
(a)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

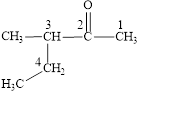
Figure 1
The longest carbon chain contains five carbon atoms. One methyl substituent is attached to the third carbon atom. The functional group present in the given compound is ketone
The structural formula is shown in Figure 1.
The correct name of the compound is
(b)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

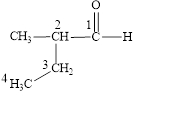
Figure 2
The longest carbon chain contains four carbon atoms. One methyl substituent is attached to the second carbon atom. The functional group present in the given compound is aldehyde
The structural formula is shown in Figure 2.
The correct name of the compound is
(c)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

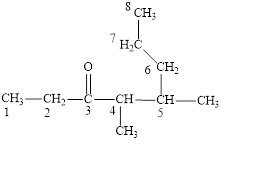
Figure 3
The longest carbon chain contains eight carbon atoms. One methyl substituent is attached to the fourth carbon atom and the other methyl group is attached to the fifth carbon atom. The functional group present in the given compound is ketone
The structural formula is shown in Figure 3.
The correct name of the compound is
(d)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

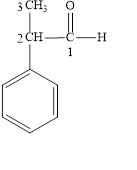
Figure 4
The longest carbon chain contains three carbon atoms. One phenyl substituent is attached to the second carbon atom. The functional group present in the given compound is aldehyde
The structural formula is shown in Figure 4.
The correct name of the compound is
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





