
Concept explainers
(a)
Interpretation:
The IUPAC name of given compound has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming ether:
- ✓ The base name is found from the longest carbon chain present in ether.
- ✓ The suffix –yl has to be changed to –oxy in order to obtain the alkoxy group name. For example, ethyl becomes as ethoxy, methyl becomes as methoxy etc.
- ✓ Alkoxy name has to be placed first with the number (carbon atom to which the alkoxy group is attached) followed by the base name.
(a)
Answer to Problem 14.103EP
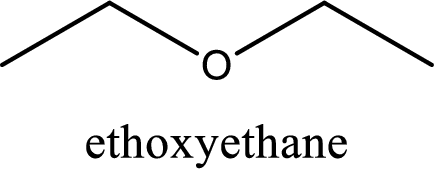
IUPAC name of the given compound is ethoxyethane.
Explanation of Solution
The structure of given compound is shown below,
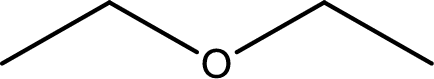
First step is to identify the longest carbon chain. In this case it is a two carbon chain. Hence, the base name is ethane.
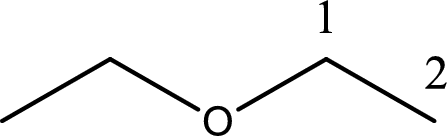
Next step is to identify the alkoxy group. In the given compound, the alkoxy group is found to be ethoxy as it contains two carbon atoms.
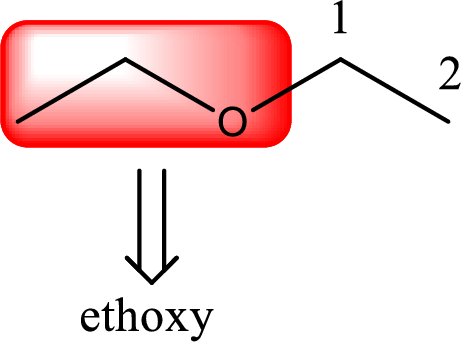
Alkoxy name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkoxy group is attached. In this case as only two carbon atoms is present and hence numbering is not required. This gives the IUPAC name as ethoxyethane.
IUPAC name for the given compound is assigned.
(b)
Interpretation:
The IUPAC name of given compound has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming ether:
- ✓ The base name is found from the longest carbon chain present in ether.
- ✓ The suffix –yl has to be changed to –oxy in order to obtain the alkoxy group name. For example, ethyl becomes as ethoxy, methyl becomes as methoxy etc.
- ✓ Alkoxy name has to be placed first with the number (carbon atom to which the alkoxy group is attached) followed by the base name.
(b)
Answer to Problem 14.103EP
IUPAC name of the given compound is 2-methoxy-2-methylpropane.
Explanation of Solution
Given structure of ether is,
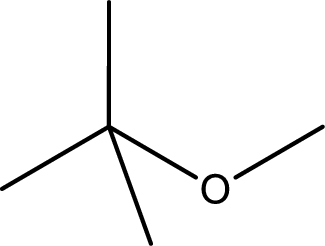
First step is to identify the longest carbon chain. In this case it is a three carbon chain. Hence, the parent
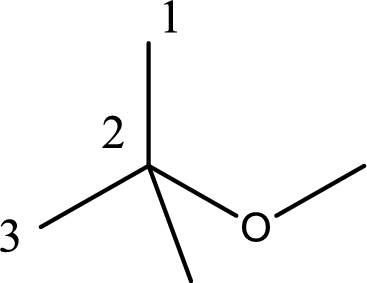
The substituent present in the longest carbon chain is a methyl group at the second carbon atom. Therefore, the base name is 2-methylpropane.
Next step is to identify the alkoxy group. In the given compound, the alkoxy group is found to be methoxy as it contains only one carbon atom.
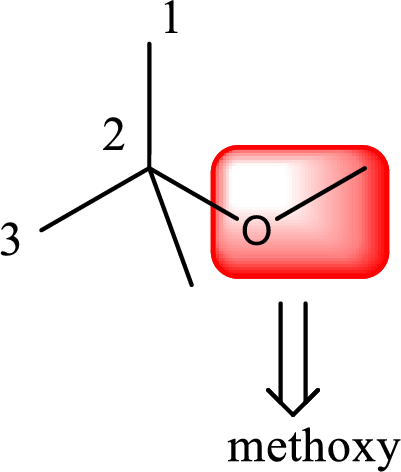
Alkoxy name is placed before the base name with the appropriate number that gives information about to which carbon atom the alkoxy group is attached. This gives the IUPAC name as 2-methoxy-2-methylpropane.
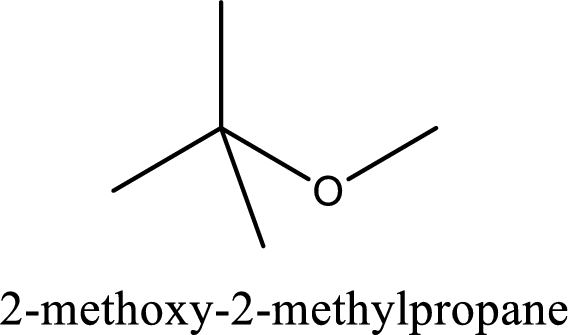
IUPAC name for the given compound is assigned.
(c)
Interpretation:
The IUPAC name of given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming alcohols that contain single hydroxyl group:
- • Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”. If the compound contains a unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the hydroxyl group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- • Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
- • If the compound contains bulky groups on same side of the double bond, then it is a cis isomer and if the bulkyl groups are present on opposite side of the double bond, then it is a trans isomer.
- • In case of cycloalkane compounds, if the substitutions are present on same side of the ring of carbon atoms, it is a cis isomer. If the substitutions are present above and below the ring, then it is a trans isomer.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
(c)
Answer to Problem 14.103EP
IUPAC name of the given compound is methoxymethanol.
Explanation of Solution
The structure of given compound is shown below,
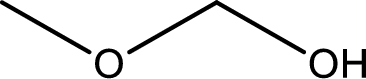
The given compound has two functional group namely, hydroxyl group and a alkoxy group. Alcohol has got more preference over ether. Therefore, the IUPAC name is given by using the parent as alcohol. In this case the parent alcohol is found to be methanol. The substituent that is present in the methanol is a methoxy group. Therefore, the IUPAC name of the given compound can be written as methoxymethanol.
IUPAC name for the given compound is assigned.
(d)
Interpretation:
The IUPAC name of given compound has to be assigned.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
IUPAC rules for naming ether:
- ✓ The base name is found from the longest carbon chain present in ether.
- ✓ The suffix –yl has to be changed to –oxy in order to obtain the alkoxy group name. For example, ethyl becomes as ethoxy, methyl becomes as methoxy etc.
- ✓ Alkoxy name has to be placed first with the number (carbon atom to which the alkoxy group is attached) followed by the base name.
(d)
Answer to Problem 14.103EP
IUPAC name of the given compound is 2-methylanisole.
Explanation of Solution
The structure of given compound is shown below,
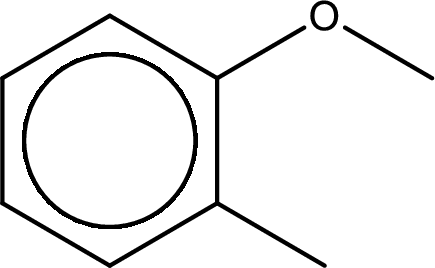
First step is to identify the longest carbon chain. In this case it is a six carbon
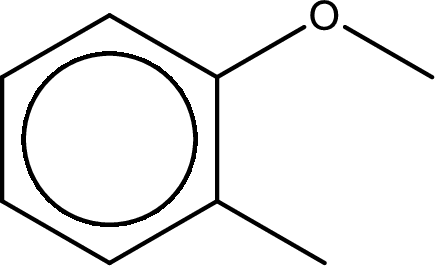
Next step is to identify the substituent present in the given structure. It is found that a methyl group is present in the second carbon atom of the aromatic benzene ring.
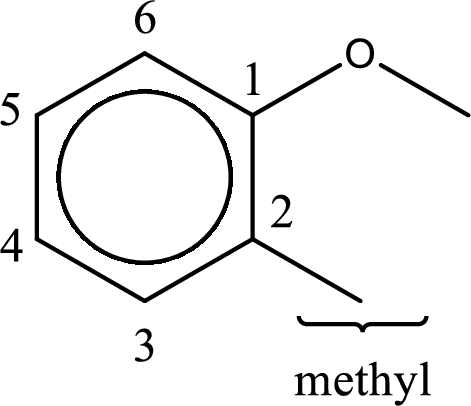
The substituent methyl group is present on the second carbon atom. The parent compound name is anisole (methoxybenzene). This gives the IUPAC name as 2-methylanisole.
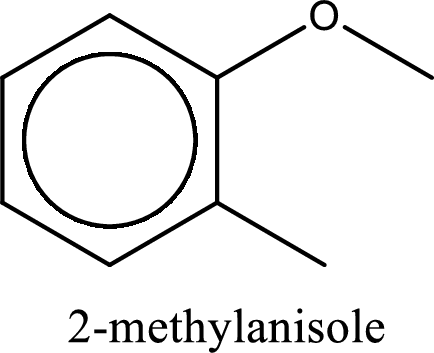
IUPAC name for the given compound is assigned.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


