
Concept explainers
An insulated tank that contains 1 kg of O2 at 15°C and 300 kPa is connected to a 2-m3 uninsulated tank that contains N2 at 50°C and 500 kPa. The valve connecting the two tanks is opened, and the two gases form a homogeneous mixture at 25°C. Determine (a) the final pressure in the tank, (b) the heat transfer, and (c) the entropy generated during this process. Assume T0 = 25°C.
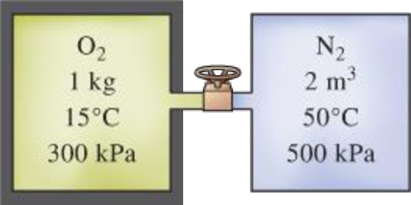
FIGURE P13–56
(a)
The pressure of the mixture.
Answer to Problem 57P
The pressure of the mixture is
Explanation of Solution
Refer to Table A-2, obtain the constant-volume specific heats of the gases at room temperature.
Write the equation to calculate the volume of the oxygen tank.
Here, mass of oxygen tank is
Calculate the mass of nitrogen gas.
Here, initial temperature and pressure of nitrogen gas is
Calculate the total volume.
Calculate the mole numbers of
Here, molar mass of
Calculate the mole number of the mixture.
Calculate the pressure of the mixture.
Here, universal gas constant of the mixture is
Conclusion:
Refer to Table A-1, obtain the gas constants of
Substitute 1 kg for
Substitute
Substitute
Refer to Table A-1, obtain the molar mass of
Substitute 1 kg for
Substitute 10.43 kg for
Substitute
Substitute
Thus, the pressure of the mixture is
(b)
The heat transfer.
Answer to Problem 57P
The heat transfer is
Explanation of Solution
Write the equation of energy balance for a closed system.
Here, heat output is
Conclusion:
Substitute 1 kg for
Thus, the heat transfer is
(c)
The entropy generation.
Answer to Problem 57P
The entropy generation is
Explanation of Solution
Write the equation of entropy balance.
Here, entropy at inlet and exit is
Calculate the mole fraction of
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Conclusion:
Substitute
Substitute
Refer to Table A-2, obtain the constant-pressure specific heats of the gases at room temperature.
Substitute 0.077 for
Substitute 0.923 for
Substitute
Thus, the entropy generation is
Want to see more full solutions like this?
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
- 4. Solve for the support reactions at A and B. W1 600 lb/ft W2 150 lb/ft A Barrow_forwardIn cold isostatic pressing, the mold is most typically made of which one of the following: thermosetting polymer tool steel sheet metal textile rubberarrow_forwardThe coefficient of friction between the part and the tool in cold working tends to be: lower higher no different relative to its value in hot workingarrow_forward
- The force F={25i−45j+15k}F={25i−45j+15k} lblb acts at the end A of the pipe assembly shown in (Figure 1). Determine the magnitude of the component F1 which acts along the member AB. Determine the magnitude of the component F2 which acts perpendicular to the AB.arrow_forwardHi can you please help me with the attached question?arrow_forwardHi can you please help me with the attached question?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





