
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.3, Problem 37P
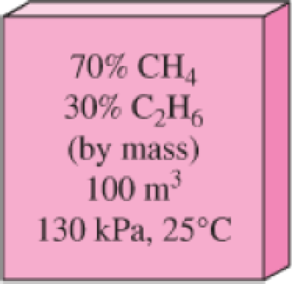
A 30 percent (by mass) ethane and 70 percent methane mixture is to be blended in a 100-m3 tank at 130 kPa and 25°C. If the tank is initially evacuated, to what pressure should ethane be added before methane is added?
FIGURE P13–39
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. The rod ABCD is made of an aluminum for which E = 70 GPa. For the loading
shown, determine the deflection of (a) point B, (b) point D.
1.75 m
Area = 800 mm²
100 kN
B
1.25 m
с
Area = 500 mm²
75 kN
1.5 m
D
50 kN
Research and select different values for the R ratio from various engine models, then analyze how these changes affect instantaneous velocity and acceleration, presenting your findings visually using graphs.
Qu. 7 The v -t graph of a car while travelling along a road is shown. Draw the s -t and a -t graphs for the motion.
I need to draw a graph and I need to show all work step by step please do not get short cut from dtna
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 6PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - Prob. 11PCh. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 18PCh. 13.3 - Prob. 19PCh. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - Prob. 26PCh. 13.3 - Prob. 27PCh. 13.3 - Prob. 28PCh. 13.3 - 13–29 A gas mixture at 350 K and 300 kPa has the...Ch. 13.3 - Prob. 30PCh. 13.3 - Prob. 31PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 33PCh. 13.3 - Prob. 34PCh. 13.3 - Prob. 35PCh. 13.3 - Prob. 36PCh. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 38PCh. 13.3 - Prob. 39PCh. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - Prob. 42PCh. 13.3 - Prob. 43PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 45PCh. 13.3 - Prob. 46PCh. 13.3 - 13–47C Is the total internal energy change of an...Ch. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - Prob. 52PCh. 13.3 - Prob. 53PCh. 13.3 - Prob. 54PCh. 13.3 - Prob. 55PCh. 13.3 - Prob. 56PCh. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - Prob. 59PCh. 13.3 - Prob. 60PCh. 13.3 - Prob. 61PCh. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 64PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 69PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 71PCh. 13.3 - Prob. 72PCh. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 80PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Prob. 83PCh. 13.3 - Prob. 84RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - Prob. 89RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 94RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - 13–102 An ideal-gas mixture consists of 2 kmol of...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 104FEPCh. 13.3 - Prob. 105FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 107FEPCh. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An unpressurized cylindrical tank with a 100-foot diameter holds a 40-foot column of water. What is total force acting against the bottom of the tank?arrow_forward7. In the following problems check to see if the set S is a vector subspace of the corresponding R. If it is not, explain why not. If it is, then find a basis and the dimension. (a) S = (b) S = {[],+,"} X1 x12x2 = x3 CR³ {[1], 4+4 = 1} CR³ X2arrow_forwardAAA Show laplace transform on 1; (+) to L (y(+)) : SY(s) = x (0) Y(s) = £ [lx (+)] = 5 x(+) · est de 2 -St L [ y (^) ] = So KG) et de D 2 D D AA Y(A) → Y(s) Ŷ (+) → s Y(s) -yarrow_forward
- 1) In each of the following scenarios, based on the plane of impact (shown with an (n, t)) and the motion of mass 1, draw the direction of motion of mass 2 after the impact. Note that in all scenarios, mass 2 is initially at rest. What can you say about the nature of the motion of mass 2 regardless of the scenario? m1 15 <+ m2 2) y "L χ m1 m2 m1 בז m2 Farrow_forward8. In the following check to see if the set S is a vector subspace of the corresponding Rn. If it is not, explain why not. If it is, then find a basis and the dimension. X1 (a) S = X2 {[2], n ≤ n } c X1 X2 CR² X1 (b) S X2 = X3 X4 x1 + x2 x3 = 0arrow_forward2) Suppose that two unequal masses m₁ and m₂ are moving with initial velocities V₁ and V₂, respectively. The masses hit each other and have a coefficient of restitution e. After the impact, mass 1 and 2 head to their respective gaps at angles a and ẞ, respectively. Derive expressions for each of the angles in terms of the initial velocities and the coefficient of restitution. m1 m2 8 m1 ↑ บา m2 ñ Вarrow_forward
- The fallowing question is from a reeds book on applied heat i am studying. Although the answer is provided, im struggling to understand the whole answer and the formulas and the steps theyre using. Also where some ov the values such as Hg and Hf come from in part i for example. Please explain step per step in detail thanks In an NH, refrigerator, the ammonia leaves the evaporatorand enters the cornpressor as dry saturated vapour at 2.68 bar,it leaves the compressor and enters the condenser at 8.57 bar with50" of superheat. it is condensed at constant pressure and leavesthe condenser as saturated liquid. If the rate of flow of the refrigerantthrough the circuit is 0.45 kglmin calculate (i) the compressorpower, (ii) the heat rejected to the condenser cooling water in kJ/s,an (iii) the refrigerating effect in kJ/s. From tables page 12, NH,:2.68 bar, hg= 1430.58.57 bar, hf = 275.1 h supht 50" = 1597.2Mass flow of refrigerant--- - - 0.0075 kgls 60Enthalpy gain per kg of refrigerant in…arrow_forwardstate the formulas for calculating work done by gasarrow_forwardExercises Find the solution of the following Differential Equations 1) y" + y = 3x² 3) "+2y+3y=27x 5) y"+y=6sin(x) 7) y"+4y+4y = 18 cosh(x) 9) (4)-5y"+4y = 10 cos(x) 11) y"+y=x²+x 13) y"-2y+y=e* 15) y+2y"-y'-2y=1-4x³ 2) y"+2y' + y = x² 4) "+y=-30 sin(4x) 6) y"+4y+3y=sin(x)+2 cos(x) 8) y"-2y+2y= 2e* cos(x) 10) y+y-2y=3e* 12) y"-y=e* 14) y"+y+y=x+4x³ +12x² 16) y"-2y+2y=2e* cos(x)arrow_forward
- The state of stress at a point is σ = -4.00 kpsi, σy = 16.00 kpsi, σ = -14.00 kpsi, Try = 11.00 kpsi, Tyz = 8.000 kpsi, and T = -14.00 kpsi. Determine the principal stresses. The principal normal stress σ₁ is determined to be [ The principal normal stress σ2 is determined to be [ The principal normal stress σ3 is determined to be kpsi. kpsi. The principal shear stress 71/2 is determined to be [ The principal shear stress 7½ is determined to be [ The principal shear stress T₁/, is determined to be [ kpsi. kpsi. kpsi. kpsi.arrow_forwardRepeat Problem 28, except using a shaft that is rotatingand transmitting a torque of 150 N * m from the left bearing to the middle of the shaft. Also, there is a profile keyseat at the middle under the load. (I want to understand this problem)arrow_forwardProb 2. The material distorts into the dashed position shown. Determine the average normal strains &x, Ey and the shear strain Yxy at A, and the average normal strain along line BE. 50 mm B 200 mm 15 mm 30 mm D ΕΙ 50 mm x A 150 mm Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
What is entropy? - Jeff Phillips; Author: TED-Ed;https://www.youtube.com/watch?v=YM-uykVfq_E;License: Standard youtube license