
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
9th Edition
ISBN: 9781266657610
Author: CENGEL
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.3, Problem 52P
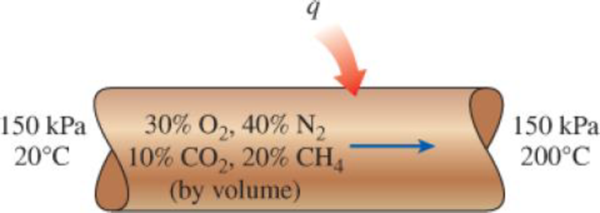
The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. This mixture is heated from 20°C to 200°C while flowing through a tube in which the pressure is maintained at 150 kPa. Determine the heat transfer to the mixture per unit mass of the mixture.
FIGURE P13–52
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sketch and desribe a balanced rudder and how it is suspended
A ship 140 m long and 18 m beam floats at a draught of9 m. The immersed cross-sectionai areas at equai intervais are 5,60, 116, 145, 152, 153, 153, 151, 142, 85 and 0 m2 respectively.Calculate:(a) displacement(b) block coefficient(c) midship section area coefficient(d) prismatic coefficient.
A steamer has waterplane area 1680m2 recorded in water with relative denisty 1.013. Displacement = 1200 t, calculate difference in draught in salwater reltive denisity 1.025.
Chapter 13 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
Ch. 13.3 - What are mass and mole fractions?Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 7PCh. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - A gas mixture consists of 20 percent O2, 30...Ch. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - Prob. 18PCh. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Prob. 22PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Prob. 24PCh. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - A gas mixture at 300 K and 200 kPa consists of 1...Ch. 13.3 - Prob. 29PCh. 13.3 - Separation units often use membranes, absorbers,...Ch. 13.3 - Prob. 31PCh. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - An engineer has proposed mixing extra oxygen with...Ch. 13.3 - A rigid tank contains 0.5 kmol of Ar and 2 kmol of...Ch. 13.3 - A mixture of gases consists of 0.9 kg of oxygen,...Ch. 13.3 - Prob. 37PCh. 13.3 - One pound-mass of a gas whose density is 0.001...Ch. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 43PCh. 13.3 - Prob. 44PCh. 13.3 - Prob. 45PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 47PCh. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - Prob. 50PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - A mixture of nitrogen and carbon dioxide has a...Ch. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - A mixture of gases consists of 0.1 kg of oxygen, 1...Ch. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - An insulated rigid tank is divided into two...Ch. 13.3 - Prob. 59PCh. 13.3 - A mixture of 65 percent N2 and 35 percent CO2...Ch. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 68PCh. 13.3 - Prob. 69PCh. 13.3 - The gas passing through the turbine of a simple...Ch. 13.3 - Prob. 71PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 79PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Is it possible for an adiabatic liquid-vapor...Ch. 13.3 - Prob. 84PCh. 13.3 - Prob. 85RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - A mixture of gases is assembled by first filling...Ch. 13.3 - Prob. 90RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 95RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - Prob. 101RPCh. 13.3 - Prob. 102FEPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 105FEPCh. 13.3 - Prob. 106FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEPCh. 13.3 - Prob. 111FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- relative velocity 11.72 m/s is correct, need help finding the angle pleasearrow_forwardDetermine the distance between the two automobiles 2 s after A has passed through the intersection.arrow_forwardA box barge 65 m long and 12 m wide floats at a draught of5.5 m in sea water. Calculate:(a) the displacement of the barge,(b) its draught in fresh waterarrow_forward
- what is the angle of the velocity of block B? velocity is 7.46 in/s Determine the acceleration and angle of block B. (Round the acceleration value to three decimal places.)arrow_forwardDetermine the relative velocity of B with respect to A.arrow_forwardGenerate the kinematic diagram of the following mechanism. Then, draw their graphs and calculate their degrees of freedom (DoF) using Gruebler's formula.arrow_forward
- An elastic bar of length L = 1m and cross section A = 1cm2 spins with angular velocity ω about an axis, as shown in the figure below. The radial acceleration at a generic point x along the bar is a(x) = ω2x, where ω= 100rad/s is the angular velocity. The bar is pinned on the rotation axis at x = 0. A mass M = 1kg is attached to the right end of the bar. Due to the radial acceleration, the bar stretches along x with displacement function u(x). The displacement u(x) solves the BVP (strong form) sketched below: d dx (σ(x)) + ρa(x) = 0 PDE σ(x) = E du dx Hooke’s law (1) u(0) =?? essential BC σ(L) =?? natural BC where σ(x) is the axial stress in the rod, ρ= 2700kg /m3 is the mass density, and E = 70GPa is the Young’s modulus 1. Define appropriate BCs for the strong BVP 2. Find the solution of the strong BVP analytically 3. Derive the weak form of the BVP.arrow_forwardGruebler's formula for the following mechanism?arrow_forwardw/I - | العنوان I need a detailed drawing with explanation SOLL эт 4 حكا The guide vane angle of a reaction turbine (Francis type make 20° with the tangent. The moving blade angle at entry is 120°. The external diameter of runner is 450 mm and the internal diameter is 300 mm. Runner width at entry is 62.5mm and at exit 100mm. Calculate the blade angle at exit for radial discharge. 96252 -20125 750 ×2.01arrow_forward
- Compressor Selection: (Q1) While a manufacturing cell is running, the calculated flow rate of air into a compressor is 40 SCFM. Which compressor from this list should be selected? A. A compressor that uses 80 SCFM B. A compressor that uses 40 SCFM C. A compressor that delivers 80 SCFM D. A compressor that delivers 40 SCFMarrow_forwardSCFM Calculation: (Q1) A pneumatic system running a manufacturing cell works on 80 psi and requires a flow rate of 10 CFM to operate. A compressor must be selected to run the cell. Calculate the amount of air going into the compressor to run this cell. (Hint: This will be in SCFM) Accurate to two decimals. Do not write the unit.arrow_forward: +00 العنوان >scóny : + 개 العنوان I need a actanicu urawing wit д い Ants nation Taxi pu +9635. The guide vane angle of a reaction turbine (Francis type make 20° with the tangent. The moving blade angle at entry is 120°. The external diameter of runner is 450 mm and the internal diameter is 300 mm. Runner width at entry is 62.5mm and at exit 100mm. Calculate the blade angle t exit for radial discharge. ۲/۱ = 44 985arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY