
Interpretation:
Charles’s Law needs to be explained.
Concept introduction:
Charles’s Law is also known as temperature-volume law.
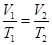
Where,
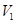 is the initial volume, and
is the initial volume, and
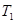 is the initial temperature, and
is the initial temperature, and
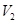 is the final volume, and
is the final volume, and
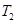 is the final temperature
is the final temperature
Explanation of Solution
According to the Charles’s Law, for a mixed mass of gas, the volume of gas decreases when temperature decreases and it increases when temperature is increased.
Charles’s Law is given by French physicist Jacques Charles. He studied how the volume of gas affects temperature on at constant pressure.
Charles’s Law declares that the volume of gas and temperature of the gas are directly proportional to each other at constant pressure.
Chapter 1 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Campbell Biology (11th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- In galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential differencearrow_forwardIf some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forwardRadiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forward
- Predict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forwardDraw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forwardDraw a curved arrow mechanism for its formation. You may need to re-draw structures to show certain bonds. Ensure that HSO is used as the base to deprotonate the ẞ carbon when necessary. C HO : OH HO: OH =s = + 1 Add/Remove step X Click and drag to start drawing a structure.arrow_forward
- Which of the following could 1,2-ethanediol be directly synthesized from? OH HO О 0 0. O ?arrow_forwardDesign a synthesis of 1,2-diethoxyethane from an alkene. Select the single best answer for each part. Part: 0/3 Part 1 of 3 Which of the following could 1,2-diethoxyethane be directly synthesized from? O HO 0 HO.... OH HO HO × 5 > ?arrow_forwardDraw the skeletal structure of the major organic product of each step of the reaction sequence. Part: 0/2 Part 1 of 2 Part: 1/2 Part 2 of 2 Continue OH NaH Na Na Br + Click and drag to start drawing a structure. X : X G : Garrow_forward
- pleasearrow_forwardplease help me please pleasearrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N2 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm ☑ 5 00. 18 Ararrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





