
Concept explainers
(a)
Interpretation:
An
Concept introduction:
E2 stands for bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the
Answer to Problem 13.35P
An alkyl halide that could have been used to synthesize the known alkene exclusively via an E2 reaction paying attention to stereochemistry is:
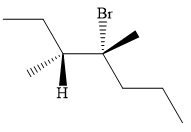
Explanation of Solution
The structure of the desired alkene is:
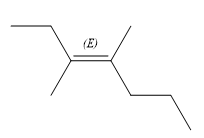
The given alkene has both the higher priority groups attached on the opposite side of the double bond. Hence, the stereochemistry is E. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(b)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
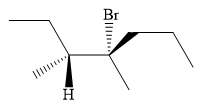
Explanation of Solution
The structure of the required alkene is:
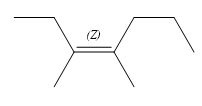
The given alkene has both the most priority groups attached on the same side of the double bond. Therefore, the stereochemistry about the double bond is Z. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(c)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
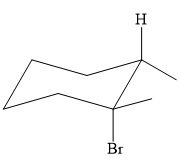
Explanation of Solution
The structure of the desired alkene is:
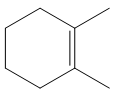
The alkene is a cycloalkene having two methyl groups attached to the double-bonded carbon atoms. As the carbon atoms in alkenes are

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(d)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
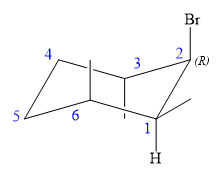
Explanation of Solution
The structure of the desired alkene is:
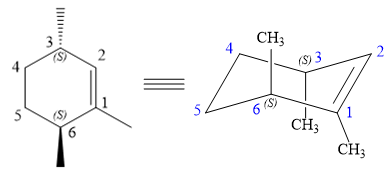
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:
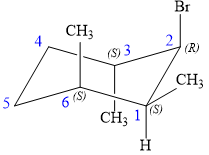
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(e)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
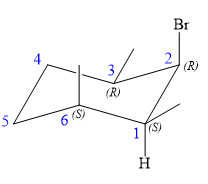
Explanation of Solution
The structure of the desired alkene is:
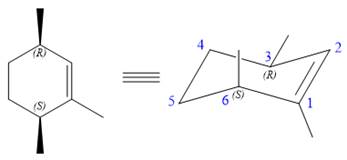
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction involves a one-step mechanism (concerted) in which carbon-halogen bond and carbon-hydrogen bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to synthesize the given alkene must be:
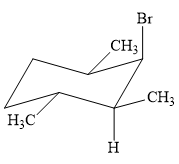
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- on x Fina X Sign X Sign x lab X Intro X Cop X chat X My x Grac x Laur x Laur x ashes x S Shox S SHE x a eve.macmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2-c stions estion. ct each urces. +95 Macmillan Learning Draw the product formed by the reaction of potassium t-butoxide with (15,25)-1-bromo-2-methyl-1-phenylbutane (shown). Clearly show the stereochemistry of the product. H BH (CH3)3CO-K+ +100 H3CW (CH3)3COH +85 H3CH2C +95 ossible ↓ Q Search Select Draw Templates More C H 0 bp A Erase 2Q 112 Resouarrow_forwardIdentify the structure of the PTH derivative generated after two rounds of Edman degradation.arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 31 0.5 30 26 29 22 28 100 27 33 26 23 15 4 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 妊 n ? Previous Nextarrow_forward
- for this question. Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forward
- Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forwardCan I please get help with this? And can I please the lowest possible significant number?arrow_forward
- What is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

