
(a)
Interpretation:
Similarities and differences in the structures of aspirin, ibuprofen and acetaminophen have to be identified.
Concept Introduction:
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
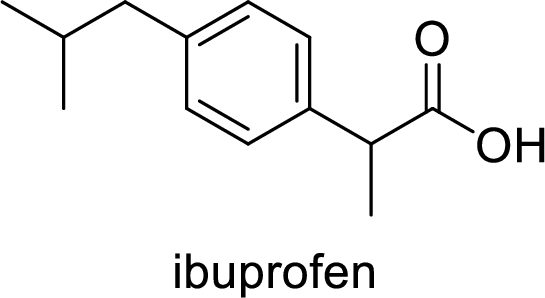
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
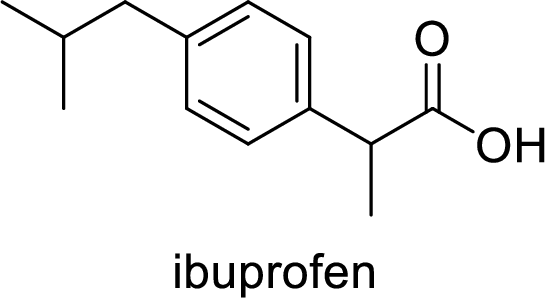
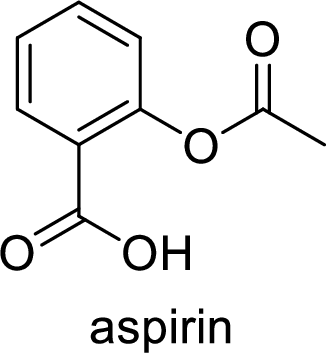
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
Alcohol: It is an organic compound where it contains at least one
(b)
Interpretation:
To find whether there is any similarities or differences in the symptoms that aspirin, ibuprofen and acetaminophen is promoted to treat have to be identified.
Concept Introduction:
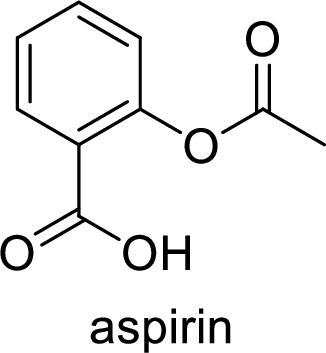
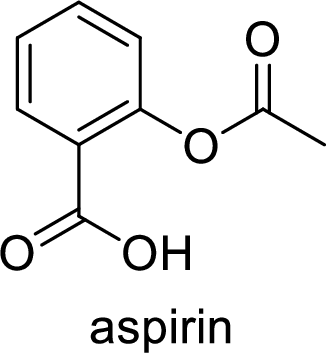
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
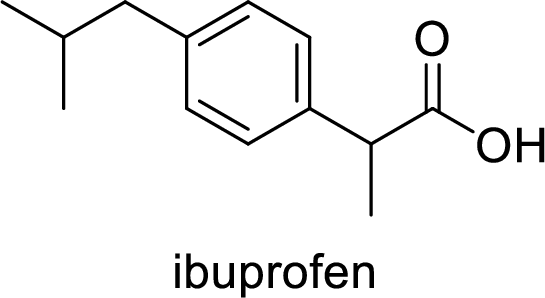
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
(b)
Interpretation:
To find whether there is any similarities or differences in the side effects of aspirin, ibuprofen and acetaminophen have to be identified.
Concept Introduction:
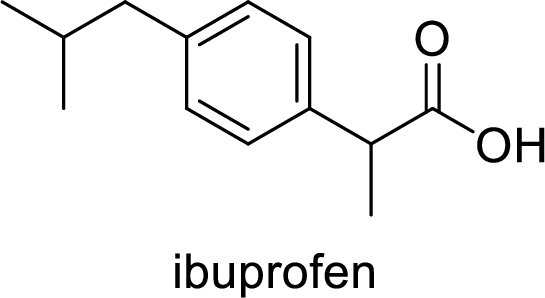
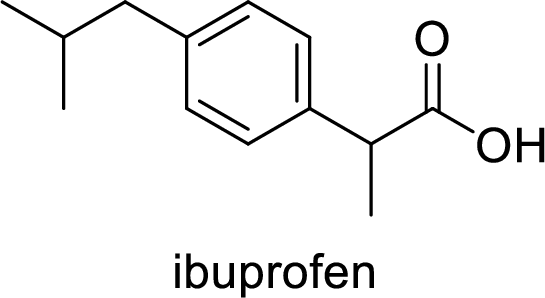
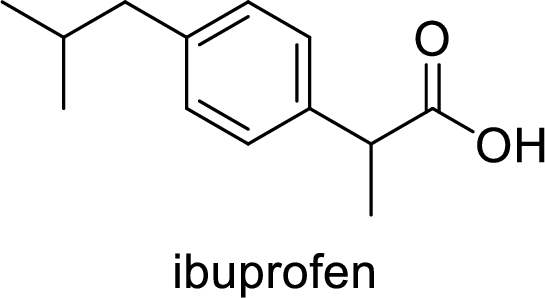
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- The structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below. How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)? Check ×arrow_forward1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forward
- Consider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forwardStudy this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forward
- calculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forwardan adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forward
- Are there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forwardhybridization of nitrogen of complex moleculesarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forward

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





