
Concept explainers
Indicate whether each of the following changes represents oxidation or reduction.
- a. FADH2 → FAD
- b. FMN → FMNH2
- c. Fe(III)SP → Fe(II)SP
- d. Cyt c1 (Fe3+) → cyt c1 (Fe2+)
(a)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
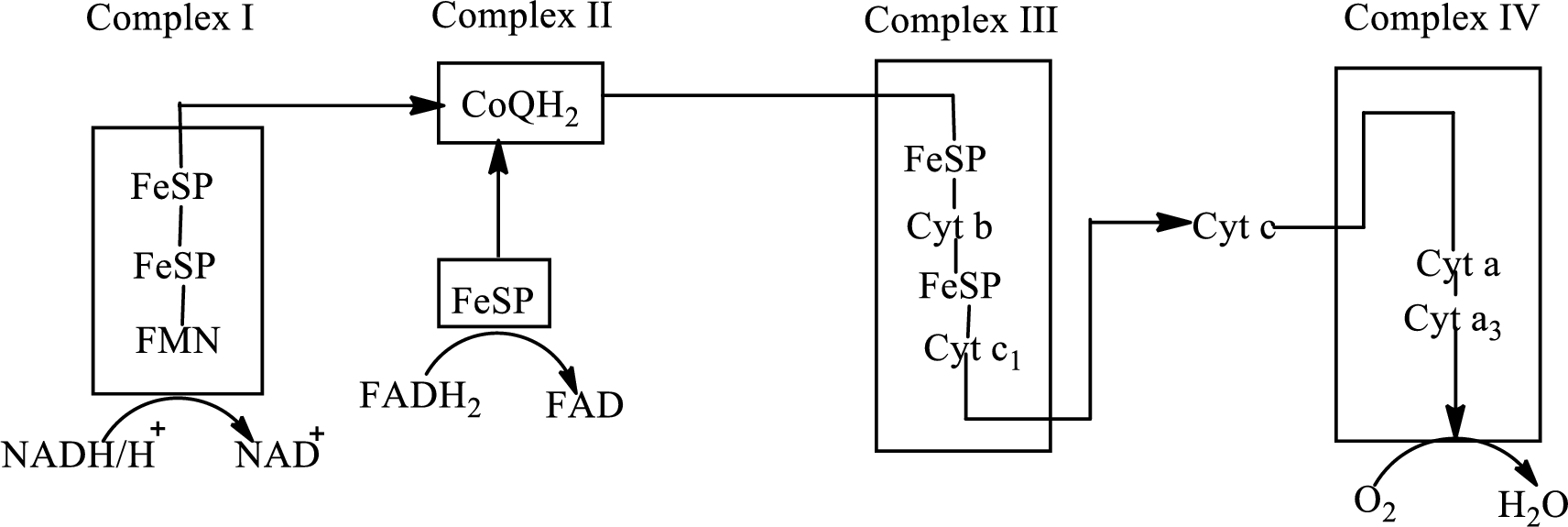
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
In the complex II, electrons are transferred from the
Here,
(b)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
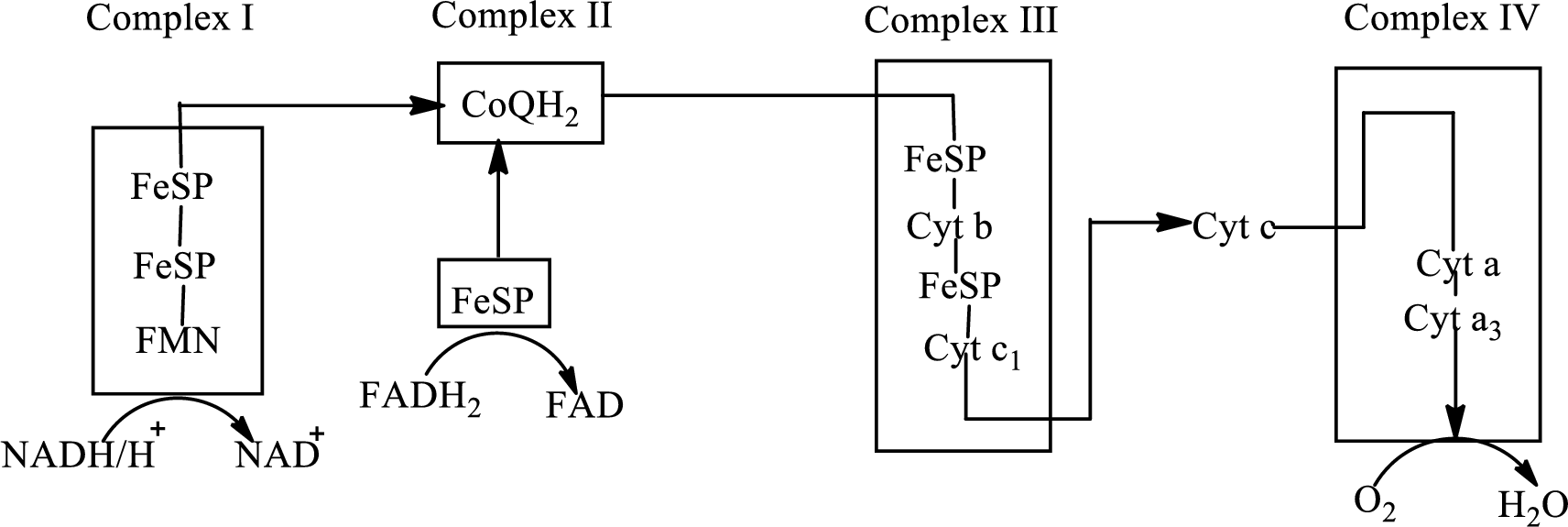
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Flavin mononucleotide is the structural component of the complex I of the electron transport chain. Flavin mononucleotide exists in two forms: FMN (oxidized form) and
Here, FMN gains electrons and hydrogen and leads to the formation of
(c)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
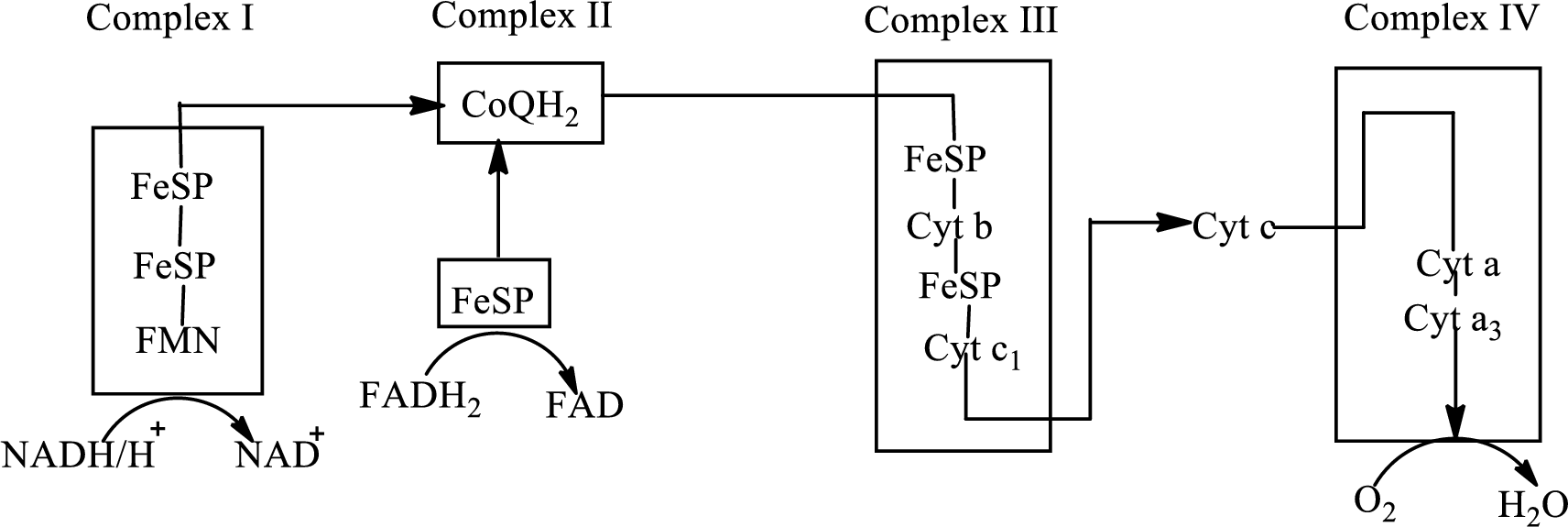
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Iron-sulfur proteins
(d)
Interpretation:
To indicate whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
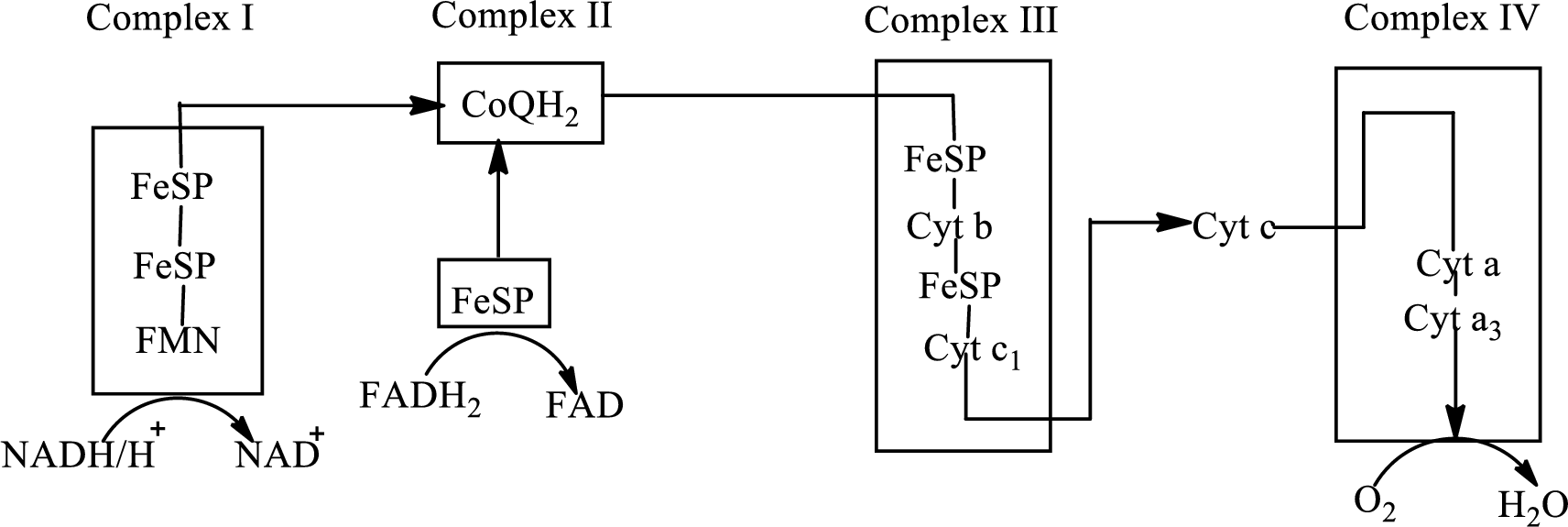
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Cytochromes are a structural component of the complex III and consist of iron that changes its oxidation state from
Want to see more full solutions like this?
Chapter 12 Solutions
Organic And Biological Chemistry
Additional Science Textbook Solutions
Physics for Scientists and Engineers
Human Biology: Concepts and Current Issues (8th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
General, Organic, and Biological Chemistry - 4th edition
Laboratory Manual For Human Anatomy & Physiology
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





