
Concept explainers
(a)
Interpretation:
Riboflavin is needed or not for the proper functioning of the CAC has to be determined.
Concept introduction:
Citric acid cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and CoA-SH. Carbon dioxide produced is exhaled out in the breathing process. The reduced form of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) is also produced. An overview of the citric acid cycle is:
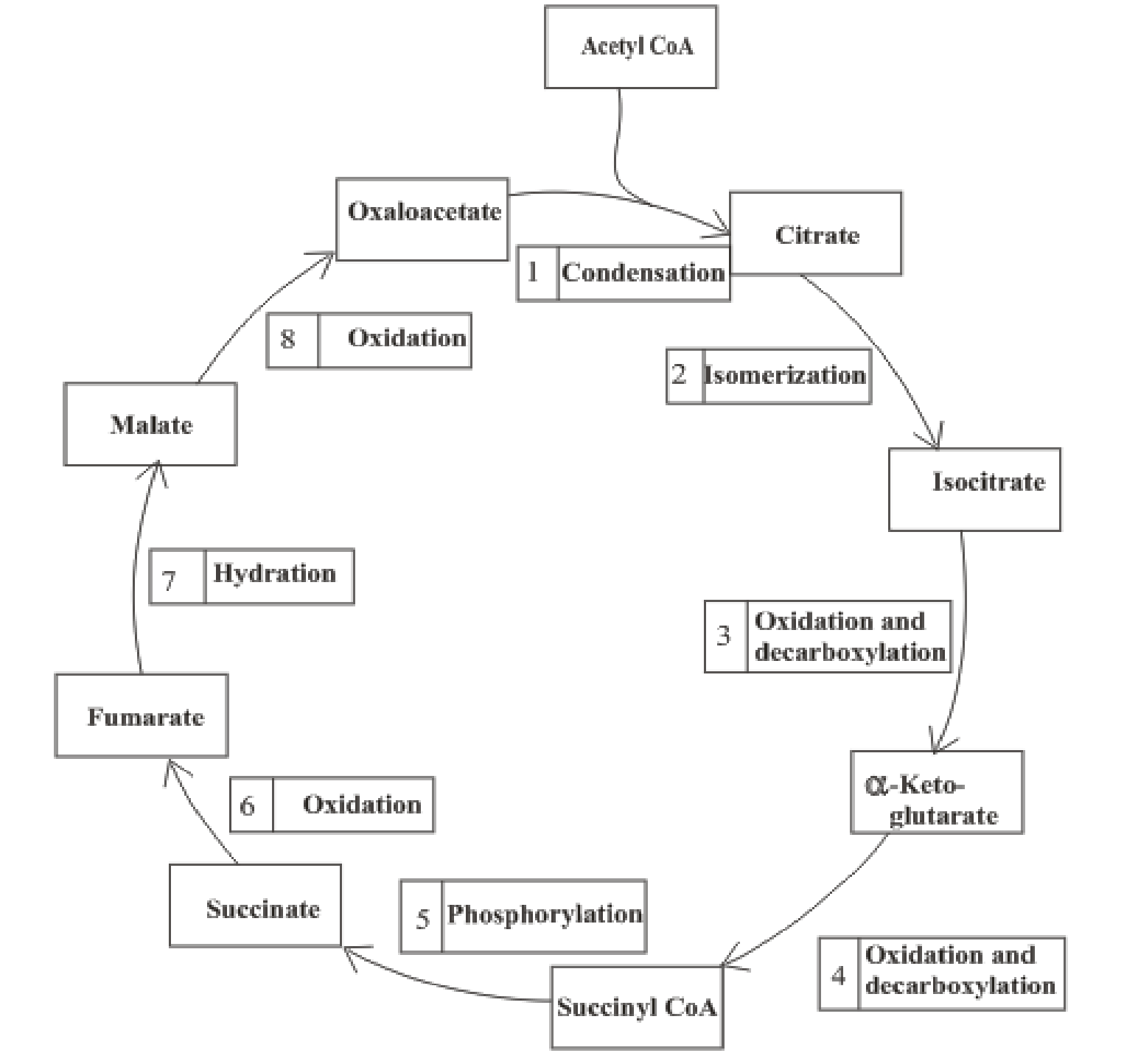
The B-vitamins consist of a group of vitamins that are water soluble and acts as precursors for enzyme cofactors.
(b)
Interpretation:
Pantothenic acid is needed or not for the proper functioning of the CAC has to be determined.
Concept introduction:
The citric acid cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and CoA-SH. Carbon dioxide produced is exhaled out in the breathing process. The reduced form of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) is also produced.
An overview of the citric acid cycle is:
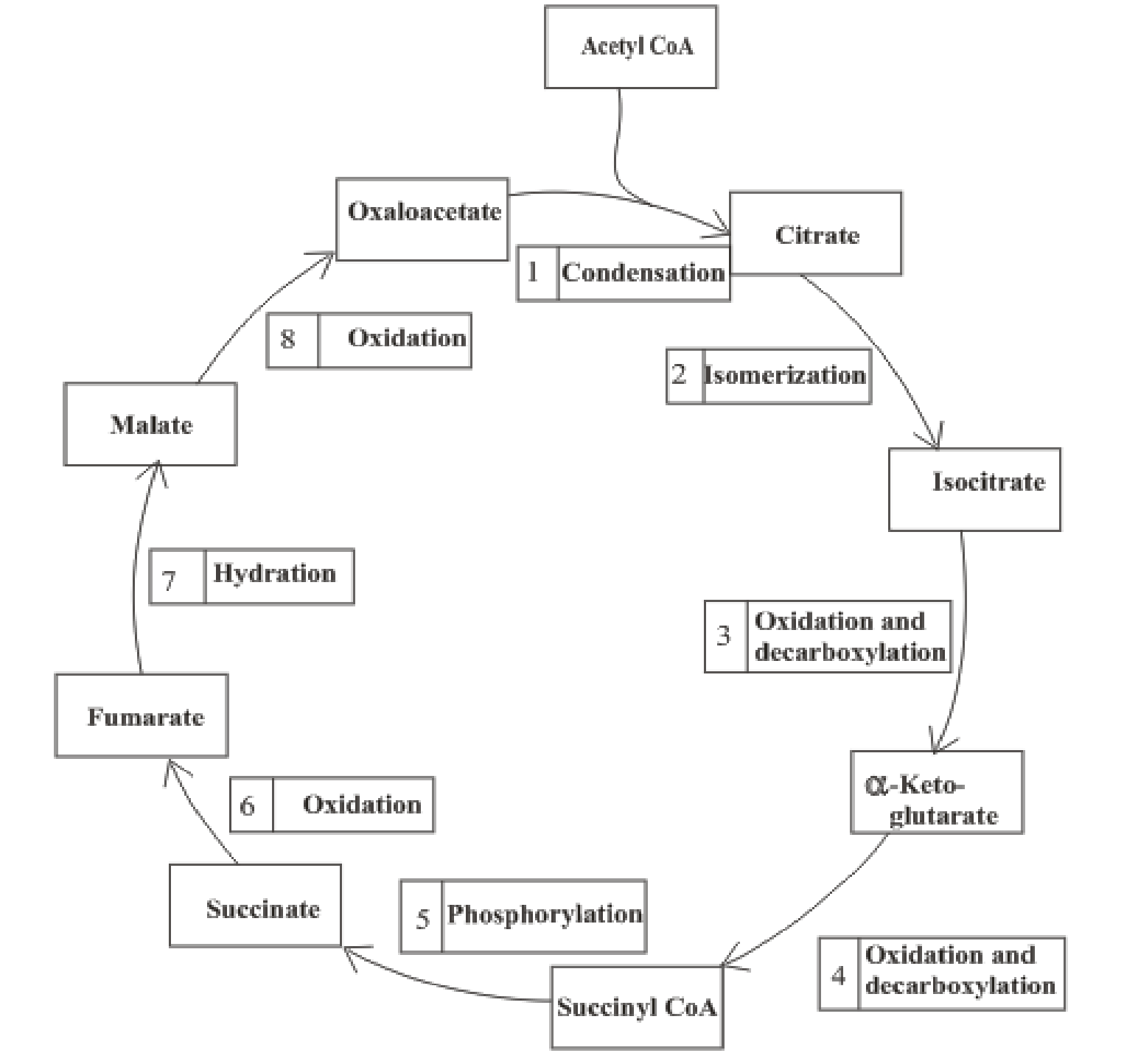
The B-vitamins consist of a group of vitamins that are water soluble and acts as precursors for enzyme cofactors.
(c)
Interpretation:
Folate is needed or not for the proper functioning of the CAC has to be determined.
Concept introduction:
The citric acid cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and CoA-SH. Carbon dioxide produced is exhaled out in the breathing process. The reduced form of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) is also produced.
An overview of the citric acid cycle is:
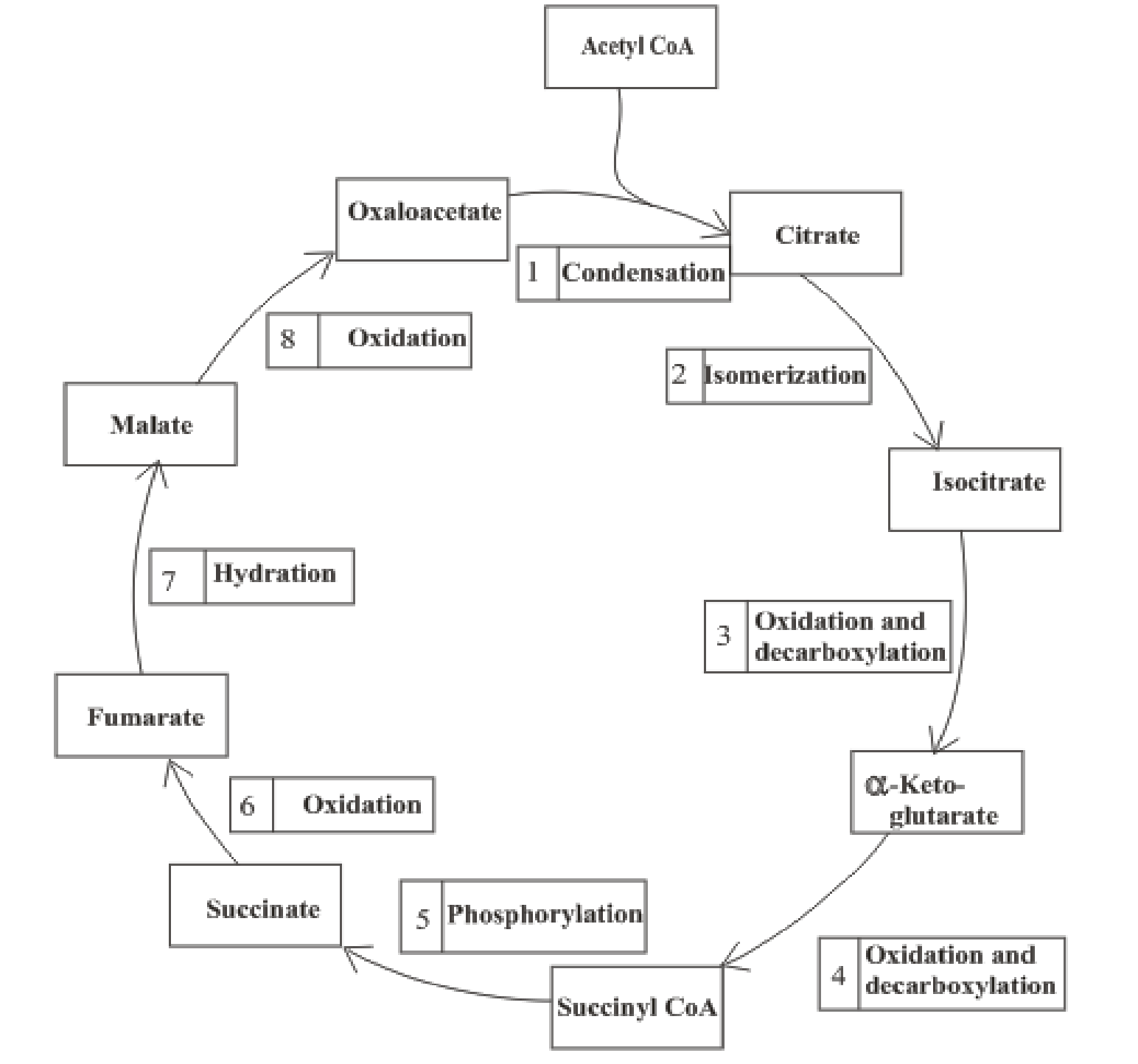
The B-vitamins consist of a group of vitamins that are water soluble and acts as precursors for enzyme cofactors.
(d)
Interpretation:
Vitamin B12 is needed or not for the proper functioning of the CAC has to be determined.
Concept introduction:
The citric acid cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and CoA-SH. Carbon dioxide produced is exhaled out in the breathing process. The reduced form of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) is also produced.
An overview of the citric acid cycle is:
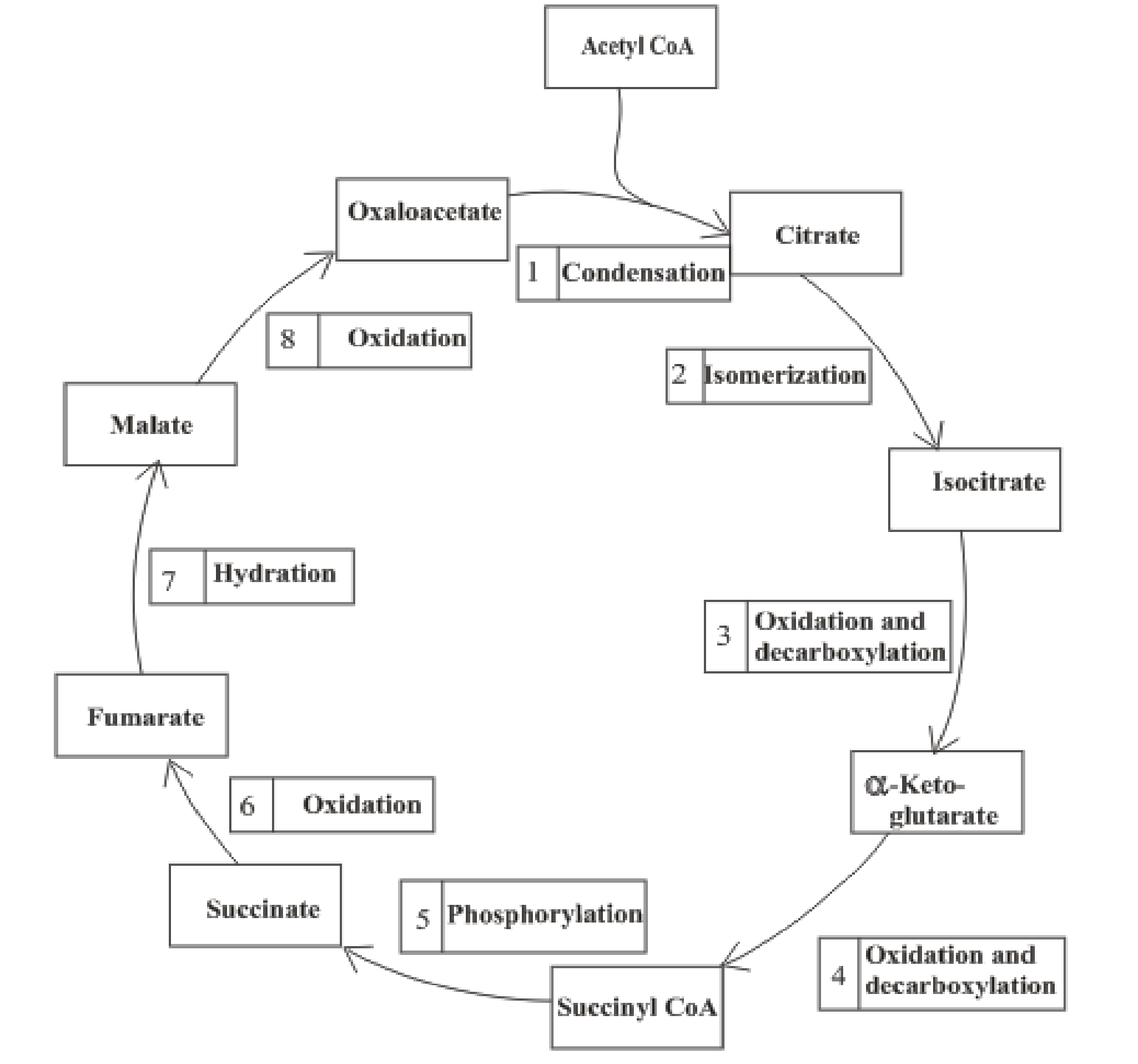
The B-vitamins consist of a group of vitamins that are water soluble and acts as precursors for enzyme cofactors.
Trending nowThis is a popular solution!

Chapter 12 Solutions
Organic And Biological Chemistry
- 3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol Please explain your steps and thought process. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4: Cl2(g) + CHCl3(g) HCl(g) + CC₁(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and 1.6 mol of CCl4. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl2 and CHCl3. ☐ x10 There will be very little HCI and CCl4. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. HCl(g)+CC14(g) 12 Cl2(9)+CHCl3(9) K = 0 ☐ What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 Cl₂(9)+2CHCl3(9) 2 HCl(9)+2CC₁₁(9) K = ✓ 00. 18 Ararrow_forward10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward
- 9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward8. When ethane thiol is treated with hydrogen peroxide the product is___.A. ethane disulfide; B. diethyl sulfide; C. ethane sulfoxide; D. ethanesulfate; E. ethyl mercaptan. Please include all steps. Thanks!arrow_forward
- 5. The major product of the three step reaction that takes place when 1-propanol is treated with strong acid is?A. dipropyl ether; B. propene; C. propanal; D. isopropyl propyl ether;E. 1-hexanol Please include all steps. Thank you!arrow_forward6. The formula of the product of the addition of HCN to benzaldehydeis ___.A. C8H7NO; B. C8H6NO; C. C14H11NO; D. C9H9NO; E. C9H8NO Please include all steps. Thank you!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid K K a name formula name formula nitrous acid HNO2 4.5×10 hydroxylamine HONH2 1.1 × 10 8 hypochlorous acid HCIO 8 3.0 × 10 methylamine CH3NH2 | 4.4 × 10¯ 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCIO solution PH choose one 0.1 M NaNO2 0.1 M CH3NH3Br 0.1 M NaBr choose one ✓ choose one v ✓ choose one 1 (lowest) ☑ 2 3 4 (highest)arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





