
Concept explainers
(a)
Interpretation:
The starting material required to prepare the given
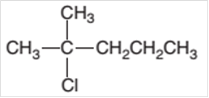
Concept Introduction:
In a
The conversion of reactant to product is represented by an arrow placed between them.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
(b)
Interpretation:
The starting material to prepare the given alkyl halide by a halogenation reaction needs to be determined.
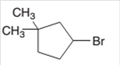
Concept Introduction:
In a chemical reaction, the conversion of a substance to new substances can be represented. The substance initially present in the reaction is reactant and the new substance formed is known as product.
The conversion of reactant to product is represented by an arrow placed between them.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
(c)
Interpretation:
The starting material to prepare the given alkyl halide by a halogenation reaction needs to be determined.
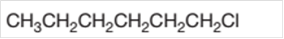
Concept Introduction:
In a chemical reaction, the conversion of a substance to new substances can be represented. The substance initially present in the reaction is reactant and the new substance formed is known as product.
The conversion of reactant to product is represented by an arrow placed between them.
Halogenation reaction is a substitution reaction in which one of the H atom of reactant hydrocarbon is substituted with halogen atom to form respective alkyl halide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
General, Organic, & Biological Chemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



