
Concept explainers
Write a balanced equation for the combustion of each sycloalkane.
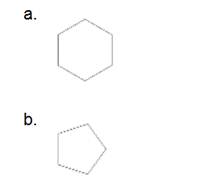
(a)
Interpretation:
The balance equation for the combustion of following cycloalkane should be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms doesn't form a ring is known as acyclic compounds.
The chemical reaction which involves the reaction of alkane and oxygen, results in the formation of carbon dioxide gas and water vapor is known as combustion reaction. The combustion reaction is an exothermic reaction.
The general reaction of combustion of alkane is:
A reaction is said to be balanced, if the number of atoms of each element on the product side and one the reactant side of a chemical reaction are equal.
Answer to Problem 12.70P
Explanation of Solution
Cyclic alkane is defined as an alkane in which series of atoms are combined to form a ring.
The general molecular formula of cyclic alkane is
The given cycloalkane is:
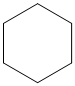
The chemical formula of the cycloalkane is
The combustion reaction of
Reactant side Product side
Number of C atoms = 6 Number of C atoms = 1
Number of H atoms = 12 Number of H atoms = 2
Number of O atoms = 2 Number of O atoms = 3
Thus, the above reaction is not balanced. To balance the reaction, multiply 9 with oxygen gas, 6 with carbon dioxide gas and 6 with water vapor.
Therefore, the balanced combustion reaction of
(b)
Interpretation:
The balance equation for the combustion of following cycloalkane should be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms doesn't form a ring is known as acyclic compounds.
The chemical reaction which involves the reaction of alkane and oxygen, results in the formation of carbon dioxide gas and water vapor is known as combustion reaction. The combustion reaction is an exothermic reaction.
The general reaction of combustion of alkane is:
A reaction is said to be balanced, if the number of atoms of each element on the product side and one the reactant side of a chemical reaction are equal.
Answer to Problem 12.70P
Explanation of Solution
Cyclic alkane is defined as an alkane in which series of atoms are combined to form a ring.
The general molecular formula of cyclic alkane is
The given cycloalkane is:
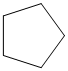
The chemical formula of the cycloalkane is
The combustion reaction of
Reactant side Product side
Number of C atoms = 5 Number of C atoms = 1
Number of H atoms = 10 Number of H atoms = 2
Number of O atoms = 2 Number of O atoms = 3
Thus, the above reaction is not balanced. To balance the reaction, multiply
Therefore, the balanced combustion reaction of
Want to see more full solutions like this?
Chapter 12 Solutions
General, Organic, & Biological Chemistry
- 14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward:0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forward
- Draw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forward
- answer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forwardTrue or false, chemistryarrow_forward
- answer thse questions with mechanisms and steps. handwritten please!arrow_forwardC app.aktiv.com Draw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CH3Br Drawingarrow_forwardDraw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CHзBr Drawingarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





