
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.2, Problem 12.6P
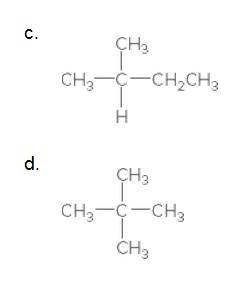
Classify the carbon atoms in each compound as 1°, 2°, 3°, or 4°.
a.
b.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 12 Solutions
General, Organic, & Biological Chemistry
Ch. 12.1 - How many hydrogen atoms are present in each...Ch. 12.1 - Which formulas represent acyclic alkanes and which...Ch. 12.2 - Prob. 12.3PCh. 12.2 - Draw two isomers with molecular formula C6H14 that...Ch. 12.2 - Prob. 12.5PCh. 12.2 - Classify the carbon atoms in each compound as 1°,...Ch. 12.2 - Prob. 12.7PCh. 12.2 - Prob. 12.8PCh. 12.2 - Prob. 12.9PCh. 12.2 - Prob. 12.10P
Ch. 12.4 - Give the IUPAC name for each compound.Ch. 12.4 - Give the IUPAC name for each compound....Ch. 12.4 - Prob. 12.13PCh. 12.4 - Prob. 12.14PCh. 12.5 - Prob. 12.15PCh. 12.5 - Prob. 12.16PCh. 12.5 - Give the IUPAC name for each compound.Ch. 12.5 - Prob. 12.18PCh. 12.7 - Answer the following questions about pentane...Ch. 12.7 - Prob. 12.20PCh. 12.7 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Prob. 12.23PCh. 12.9 - Prob. 12.24PCh. 12.9 - Prob. 12.25PCh. 12.9 - Prob. 12.26PCh. 12 - Prob. 12.27PCh. 12 - Prob. 12.28PCh. 12 - Prob. 12.29PCh. 12 - Prob. 12.30PCh. 12 - Classify each carbon as 1°, 2°, 3°, or 4°. a....Ch. 12 - Prob. 12.32PCh. 12 - Label each pair of compounds as constitutional...Ch. 12 - Label each pair of compounds as constitutional...Ch. 12 - Consider compounds A, B, and C. Label each pair of...Ch. 12 - Consider compounds D,E, and F. Label each pair of...Ch. 12 - Prob. 12.37PCh. 12 - Prob. 12.38PCh. 12 - Draw structures that fit the following...Ch. 12 - Draw the five constitutional isomers having...Ch. 12 - Prob. 12.41PCh. 12 - Prob. 12.42PCh. 12 - Prob. 12.43PCh. 12 - Prob. 12.44PCh. 12 - Prob. 12.45PCh. 12 - Prob. 12.46PCh. 12 - Prob. 12.47PCh. 12 - Prob. 12.48PCh. 12 - Prob. 12.49PCh. 12 - Prob. 12.50PCh. 12 - Give the IUPAC name for each cycloalkane.Ch. 12 - Prob. 12.52PCh. 12 - Prob. 12.53PCh. 12 - Give the structure corresponding to each IUPAC...Ch. 12 - Each of the following IUPAC names is incorrect....Ch. 12 - Each of the following IUPAC names is incorrect....Ch. 12 - Draw three constitutional isomers having molecular...Ch. 12 - Draw four constitutional isomers having molecular...Ch. 12 - Draw a skeletal structure for each compound octane...Ch. 12 - Convert each compound to a skeletal structure CH3(...Ch. 12 - Convert each skeletal structure to a complete...Ch. 12 - Convert each skeletal structure to a complete...Ch. 12 - Which compound in each pair has the higher melting...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Branching in an alkane chain decreases surface...Ch. 12 - Explain why the boiling points of heptane [CH3( CH...Ch. 12 - Explain why hexane is more soluble in...Ch. 12 - Mineral oil and Vaseline are both mixtures of...Ch. 12 - Write a balanced equation for the combustion of...Ch. 12 - Write a balanced equation for the combustion of...Ch. 12 - Write a balanced equation for the incomplete...Ch. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - Prob. 12.75PCh. 12 - Prob. 12.76PCh. 12 - Prob. 12.77PCh. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - Prob. 12.80PCh. 12 - Prob. 12.81PCh. 12 - Prob. 12.82PCh. 12 - Prob. 12.83PCh. 12 - A major component of animal fat is tristearin, (a)...Ch. 12 - Answer the following questions about the alkane...Ch. 12 - Prob. 12.86PCh. 12 - Prob. 12.87PCh. 12 - Answer the questions in Problem 12.85 for the...Ch. 12 - Prob. 12.89CPCh. 12 - Draw the structure of the 12 constitutional...Ch. 12 - Cyclopentane has a higher boiling point than...Ch. 12 - Prob. 12.92CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY