
(a)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkene used to synthesize the given compound is
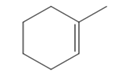
The necessary reagents and special reaction conditions for the synthesis are
Explanation of Solution
The structure of the given compound is
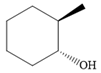
Alcohol with a specific stereochemistry is to be synthesized from an alkene. A reaction involving a carbocation needs to be avoided to prevent unwanted carbocation rearrangements. Also, the OH group must be added to a less substituted carbon, i.e., an anti-Markovnikov addition is needed. Therefore, the reaction needs to be carried out using hydroboration-oxidation.
The appropriate alkene for the synthesis of the given compound would be
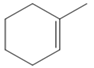
The given compound is synthesized by using the above alkene via hydroboration-oxidation reaction. So the necessary reagents for the reaction are
In the first step, an electrophilic addition of borane across the double bond of the alkene takes place either from above or below the plane of the alkene. So a mixture of enantiomers is obtained after oxidation of the adduct by basic
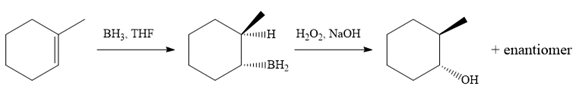
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(b)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkyne used to synthesize the given compound is
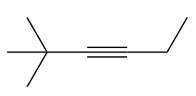
The necessary reagents and special reaction conditions to synthesize the given compound are
Explanation of Solution
The given compound is
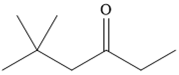
It is a ketone, so the starting compound must be an alkyne. A hydroboration-oxidation reaction can convert an alkyne into a ketone. Since only one molecule of borane is to be added, a bulky reagent like disiamylborane is more appropriate than borane. Also, the dialkylborane part must add to the less hindered carbon of the triple bond. Therefore, the triple bond must be between the carbon bonded to oxygen and the carbon close to the bulky tertiary carbon. Therefore, the alkyne that can be used is

A sterically hindered dialkylborane, like disiamylborane
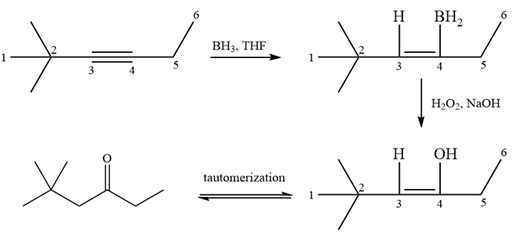
Thus the specific reagents and reaction conditions are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(c)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkyne used to synthesize the given compound is
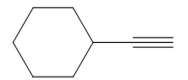
The necessary reagents and special reaction conditions to synthesize the given compound are
Explanation of Solution
The given compound is
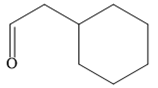
It is an aldehyde, so it can be prepared from a terminal alkyne by hydroboration-oxidation. In the hydroboration reaction, boron is added to the terminal carbon. So the appropriate alkyne for the synthesis of the given compound is
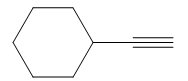
The alkyne is treated with the bulky disiamylborane to prevent the addition of a second molecule and formation of a mixture of products. Subsequent treatment of the adduct by
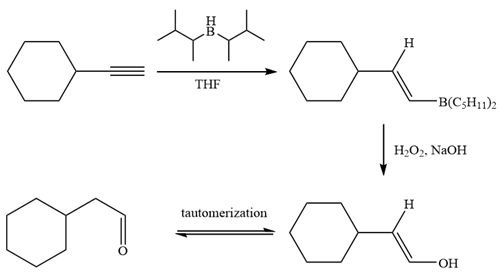
Thus, the necessary reagents and special reaction conditions for the synthesis are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(d)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkene used to synthesize the given compound is
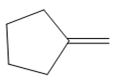
The necessary reagents and special reaction conditions for the synthesis are
Explanation of Solution
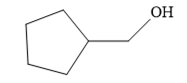
Since the product is an alcohol, an alkene with a methylene substituent on a cyclopentane ring would be appropriate as the starting compound.

Treating this alkene with borane in THF will add
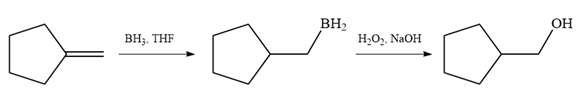
Thus, the necessary reagents for the reaction are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- CH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward2.0arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forwardA certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward
- 2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forwardCommercial bleach contains either chlorine or oxygen as an active ingredient. A commercial oxygenated bleach is much safer to handle and less likely to ruin your clothes. It is possible to determine the amount of active ingredient in an oxygenated bleach product by performing a redox titration. The balance reaction for such a titration is: 6H+ +5H2O2 +2MnO4- à 5O2 + 2Mn2+ + 8H2O If you performed the following procedure: “First, dilute the Seventh Generation Non-Chlorine Bleach by pipetting 10 mL of bleach in a 100 mL volumetric flask and filling the flask to the mark with distilled water. Next, pipet 10 mL of the diluted bleach solution into a 250 mL Erlenmeyer flask and add 20 mL of 1.0 M H2SO4 to the flask. This solution should be titrated with 0.0100 M KMnO4 solution.” It took 18.47mL of the KMnO4 to reach the endpoint on average. What was the concentration of H2O2 in the original bleach solution in weight % assuming the density of bleach is 1g/mL?arrow_forward
- 10.arrow_forwardProper care of pH electrodes: Why can you not store a pH electrode in distilled water? What must you instead store it in? Why?arrow_forwardWrite the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 569 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! §arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





