
Concept explainers
(a)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting
Answer to Problem 12.26P
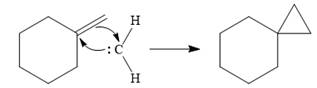
The mechanism for the reaction when
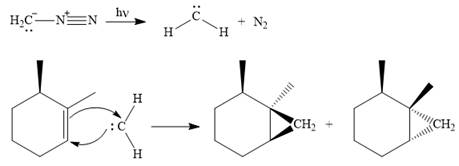
Explanation of Solution
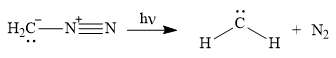
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
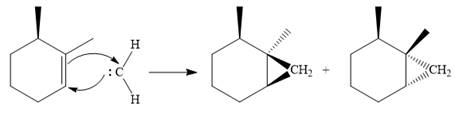
Since the starting alkene is chiral, the product will be a mixture of two enantiomers. The pair of enantiomers is produced because the carbene can add either above or below the plane of the ring in the reactant.
Thus the complete mechanism for this reaction can be drawn as
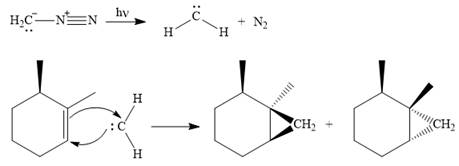
A carbene adds to an alkene or
(b)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
The mechanism for the given reaction is
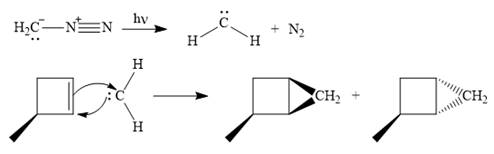
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.

The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
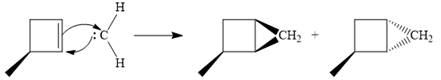
Since the starting alkene is chiral, the product will be a mixture of two enantiomers. The pair of enantiomers is produced because the carbene can add either above or below the plane of the ring in the reactant.
Thus the complete mechanism for this reaction can be drawn as:
A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(c)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
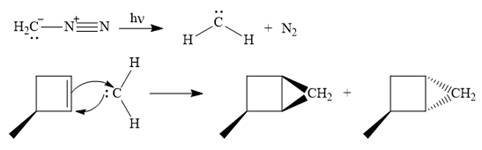
The mechanism for the given reaction is
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
Only one product is formed in this case because of the symmetry of the reactant alkene about the axis of the double bond.
Thus, the complete mechanism for the given reaction can be drawn as
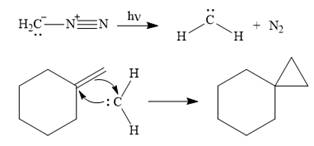
A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(d)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
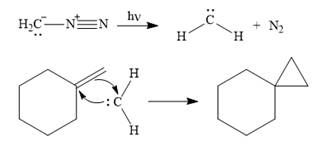
The mechanism for the given reaction is
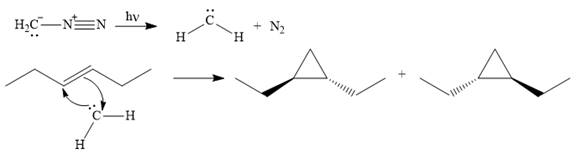
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
The starting alkene has a trans geometry, therefore, the two substituents on the cyclopropane ring in the product are on opposite sides of the ring.
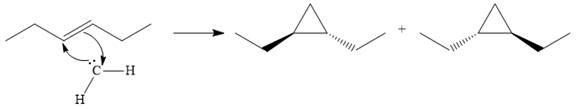
This means the product is chiral, and a racemic mixture of two enantiomers will be produced.
Thus, the complete mechanism for the reaction can be drawn as

A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(e)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
The mechanism for the given reaction is
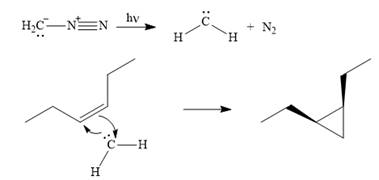
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
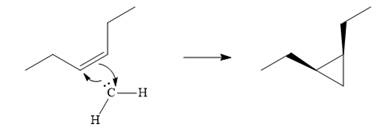
The starting alkene has a cis geometry and is symmetric. Therefore, only one product, a meso compound is produced.
Thus the complete mechanism for this reaction can be drawn as

A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
- 3. Name this compound properly, including stereochemistry. H₂C H3C CH3 OH 4. Show the step(s) necessary to transform the compound on the left into the acid on the right. Bri CH2 5. Write in the product of this LiAlH4 Br H₂C OHarrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
- A block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


