
Concept explainers
What
or
a. ![Chapter 12, Problem 12.44P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 1](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image001.jpg) b.
b. ![Chapter 12, Problem 12.44P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 2](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image002.jpg) c.
c. ![Chapter 12, Problem 12.44P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 3](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image003.jpg)
(a)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.44P
The alkene needed to synthesize the given
Explanation of Solution
In presence of
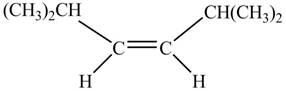
Figure 1
The corresponding chemical reaction is given below.
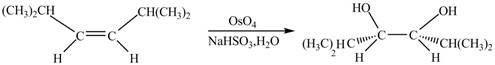
Figure 2
In presence of
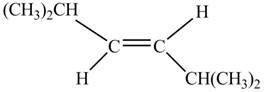
Figure 3
The corresponding chemical reaction is given below.
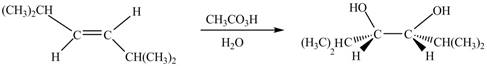
Figure 4
The alkene needed to synthesize the given
(b)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.44P
The alkene needed to synthesize the given
Explanation of Solution
In presence of
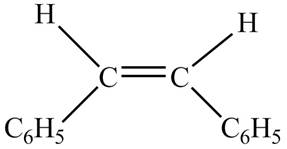
Figure 5
The chemical reaction is given below.
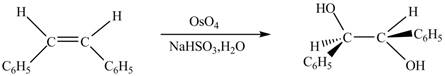
Figure 6
In presence of
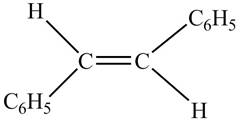
Figure 7
The chemical reaction is given below.
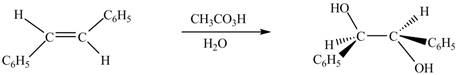
Figure 8
The alkene needed to synthesize the given
(c)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.44P
The alkene needed to synthesize the given
Explanation of Solution
The given alkene is,
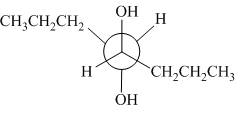
Figure 9
This is a staggered conformation in which two hydroxyl groups are anti to each other.
In presence of
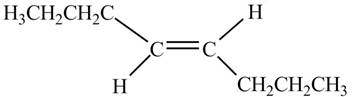
Figure 10
The chemical reaction is given below.
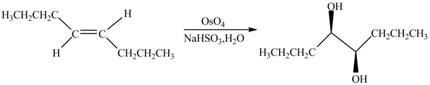
Figure 11
In presence of
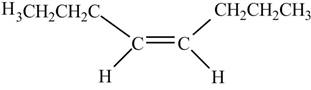
Figure 12
The chemical reaction is given below.
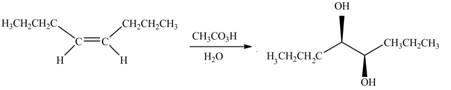
Figure 13
The alkene needed to synthesize the given
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Campbell Biology (11th Edition)
Organic Chemistry (8th Edition)
Chemistry: Structure and Properties (2nd Edition)
Biochemistry: Concepts and Connections (2nd Edition)
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
