
Draw the organic products formed when cyclopentene is treated with each reagent. With some
reagents, no reaction occurs.
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
k.
l. Product in (k);
(a)
Interpretation: The organic product formed when cyclopentene is treated with
Concept introduction: The addition of
Answer to Problem 12.38P
The organic product formed when cyclopentene
Explanation of Solution
In the given reaction, when cyclopentene is treated with
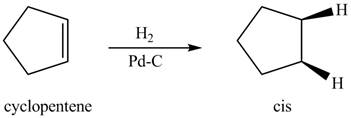
Figure 1
The organic product formed when cyclopentene
(b)
Interpretation: The organic product formed when cyclopentene is treated with Lindlar catalyst is to be predicted.
Concept introduction: The addition of
Answer to Problem 12.38P
Cyclopentene do not react with Lindlar catalyst.
Explanation of Solution
Alkenes do not react with Lindlar catalyst. Lindlar catalyst is used to reduce alkynes to
Cyclopentene do not react with Lindlar catalyst.
(c)
Interpretation: The product formed when the cyclopentene is treated with
Concept introduction: In presence of sodium metal in ammonia, the is alkyne is reduced to
Answer to Problem 12.38P
Cyclopentene do not react with
Explanation of Solution
Alkenes do not react with
Cyclopentene do not react with
(d)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
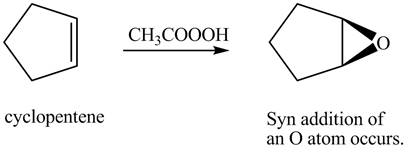
Figure 2
The product formed when cyclopentene is treated with
(e)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Anti-dihroxylation takes place in two steps epoxidation followed by hydrolysis. In presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
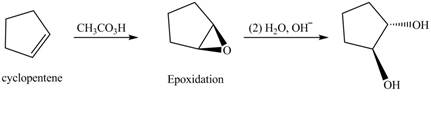
Figure 3
The product formed when cyclopentene is treated with
(f)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Addition of two hydroxyl groups on double bond to form
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
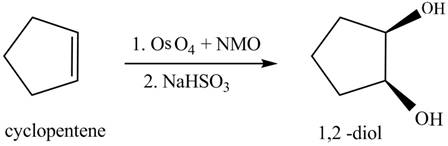
Figure 4
The product formed when cyclopentene is treated with
(g)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Addition of two hydroxyl groups on double bond to form
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
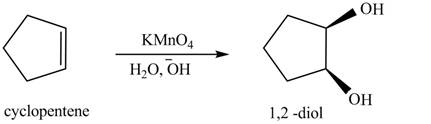
Figure 5
The product formed when cyclopentene is treated with
(h)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: The metal hydride reagents are good reducing agents such as
Answer to Problem 12.38P
Cyclopentene gives no reaction with
Explanation of Solution
The
Cyclopentene gives no reaction with
(i)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of ozone and
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
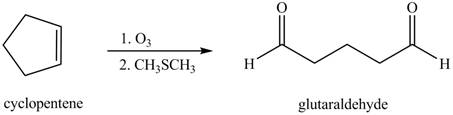
Figure 6
The product formed when cyclopentene is treated with
(j)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Sharpless epoxidation involves the oxidation of double bond between carbon atoms to epoxide. This oxidation occurs only in allylic alcohol. This is an enantioselective oxidation, which means predominantly one enantiomer is formed. Sharpless reagents are
Answer to Problem 12.38P
The product formed when cyclopenteneis treated with
Explanation of Solution
There are two different chiral diethyl tartrate isomers,
When epoxidation is done using
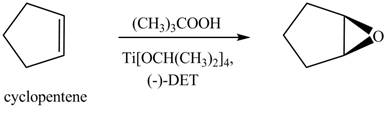
Figure 7
The product formed when cyclopenteneis treated with
(k)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide alkene is oxidized to epoxide this is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
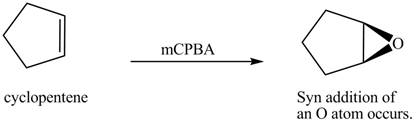
Figure 8
The product formed when cyclopentene is treated with
(l)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide, alkene is oxidized to epoxide this is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
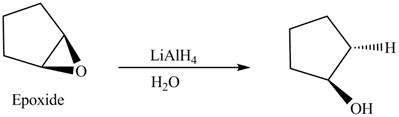
Figure 9
The product formed when cyclopentene is treated with
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
Brock Biology of Microorganisms (15th Edition)
Microbiology Fundamentals: A Clinical Approach
Human Physiology: An Integrated Approach (8th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
- If the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





