
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 12.32P
Label each reaction as oxidation, reduction, or neither.
a. 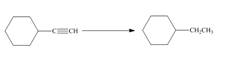 c.
c. 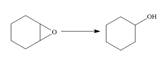
b.
![]()
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O.
The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.
What is the structure of the monomer?
→
BINDERIYA GANBO... BINDERIYA GANBO.
AP Biology Notes
Gamino acid chart - G...
36:22
司
10
☐ Mark for Review
Q
1
Hide
80
8
2
=HA O=A¯ = H₂O
Acid
HIO
HBrO
HCIO
Question 10 of 35 ^
Σ
DELL
□
3
%
Λ
&
6
7
* ∞
8
do 5
$ 4
# m
3
°
(
9
Highlights & Notes
AXC
Sign out
C
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 12.1PCh. 12 - Prob. 12.2PCh. 12 - Prob. 12.3PCh. 12 - Prob. 12.4PCh. 12 - Prob. 12.5PCh. 12 - Given that syn addition of H2 occurs from both...Ch. 12 - Compound Molecular formula before...Ch. 12 - Draw the products formed when triacylglycerol A is...Ch. 12 - Prob. 12.9PCh. 12 - Prob. 12.10P
Ch. 12 - Problem 12.11 (a) Draw the structure of a compound...Ch. 12 - Prob. 12.12PCh. 12 - Prob. 12.13PCh. 12 - Prob. 12.14PCh. 12 - Prob. 12.15PCh. 12 - Prob. 12.16PCh. 12 - Prob. 12.17PCh. 12 - Problem 12.18 Draw the products formed when both...Ch. 12 - Prob. 12.19PCh. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - Prob. 12.22PCh. 12 - Prob. 12.23PCh. 12 - Problem 12.24 Draw the organic products in each of...Ch. 12 - Prob. 12.25PCh. 12 - Prob. 12.26PCh. 12 - Problem 12.27 Draw the products of each Sharpless...Ch. 12 - Prob. 12.28PCh. 12 - 12.29 Draw the products formed when A is treated...Ch. 12 - Prob. 12.30PCh. 12 - 12.31 Devise a synthesis of the following compound...Ch. 12 - Label each reaction as oxidation, reduction, or...Ch. 12 - Prob. 12.33PCh. 12 - Prob. 12.34PCh. 12 - Prob. 12.35PCh. 12 - Prob. 12.36PCh. 12 - 12.37 Stearidonic acid (C18H28O2) is an...Ch. 12 - Draw the organic products formed when cyclopentene...Ch. 12 - Prob. 12.39PCh. 12 - Draw the organic products formed when allylic...Ch. 12 - Draw the organic products formed in each reaction...Ch. 12 - Draw the organic products formed in each reaction....Ch. 12 - Prob. 12.43PCh. 12 - Prob. 12.44PCh. 12 - Prob. 12.45PCh. 12 - What alkene is needed to synthesize each 1,2-diol...Ch. 12 - Prob. 12.47PCh. 12 - Draw the products formed after Steps 1 and 2 in...Ch. 12 - Prob. 12.49PCh. 12 - Prob. 12.50PCh. 12 - Prob. 12.51PCh. 12 - What alkyne gives each set of products after...Ch. 12 - Prob. 12.53PCh. 12 - Prob. 12.54PCh. 12 - Prob. 12.55PCh. 12 - 12.54 An unknown compound A of molecular formula ...Ch. 12 - 12.55 DHA is a fatty acid derived from fish oil...Ch. 12 - Prob. 12.58PCh. 12 - Prob. 12.59PCh. 12 - 12.58 Epoxidation of the following allylic alcohol...Ch. 12 - What allylic alcohol and DET isomer are needed to...Ch. 12 - Devise a synthesis of each hydrocarbon from...Ch. 12 - Prob. 12.63PCh. 12 - 12.62 It is sometimes necessary to isomerize a cis...Ch. 12 - Prob. 12.65PCh. 12 - Prob. 12.66PCh. 12 - Prob. 12.67PCh. 12 - Prob. 12.68PCh. 12 - Devise a synthesis of each compound from the...Ch. 12 - Devise a synthesis of each compound from acetylene...Ch. 12 - Prob. 12.71PCh. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - Prob. 12.75PCh. 12 - Prob. 12.76PCh. 12 - 12.72 Draw a stepwise mechanism for the following...Ch. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - Prob. 12.80PCh. 12 - 12.75 Sharpless epoxidation of allylic alcohol X...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
- In the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward
- 18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forward
- The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY