
Concept explainers
(a)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
Reduction of an
Answer to Problem 12.43P
The reagents needed to carry out the transformation is H2/Pd-C to form the product

Explanation of Solution
Reduction of an alkene by the addition of H2 to one or both of the pi bonds. When an alkene is treated with two or more equivalents of H2 and using the Pd catalyst, reduction of both the pi bonds occurs. Syn addition of one equivalent of H2 to forms a cis alkane product. Thus one more addition of a equivalent of H2 to form an alkane. Four new C-H bonds are formed.
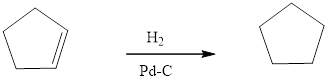
Reduction of the given transformation is carried out by using the Hydrogen (1 equ)/Pd-C reagent
(b)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is obtained by epoxidation, it is a stereospecific reaction because cis and trans alkenes yield different stereoisomers as products. A cis alkene gives an
Answer to Problem 12.43P
The reagents needed to carry out the transformation is mCPBA or metachloro perbenzoic acid to form the product
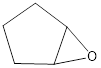
Explanation of Solution

Two C-O bonds are formed to one oxygen atom with one electron pair from the peroxyacid and one from the pi bond. Then the weak oxygen-oxygen bond is broken to form the epoxide product.

Reduction of the given transformation is carried out by using the mCPBA or metachloro perbenzoic acid reagent.
(c)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is dihydroxylation product. It is the addition of two hydroxy groups to a double bond, forming a 1, 2-
Answer to Problem 12.43P
The reagents needed to carry out the transformation is OsO4, NaHSO3+H2O or KMnO4+H2O to form the product
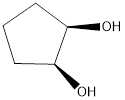
Explanation of Solution
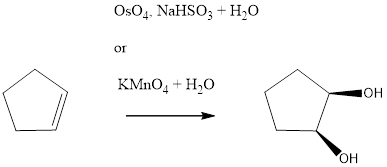
The reagent adds two oxygen atoms to the same side of the double bond in a syn fashion-to yield a cyclic intermediate. The cyclic intermediate is then to hydrolysis to cleaves the metalo oxygen bonds, forming the cis-1, 2-diol. With OsO4, sodium bisulfite (NaHSO3) is added for the hydrolysis to occur. In KMnO4 only H2O is used.
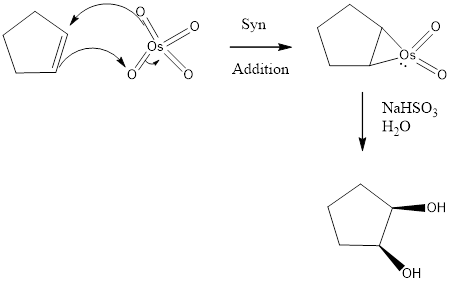
Reduction of the given transformation is carried out by using the OsO4, NaHSO3+H2O or KMnO4+H2O reagent.
(d)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is reduction product. Reduction of these C-X sigma bonds is an example of nucleophilic substitution, in which LiAlH4 serves as a source of a hydride nucleophile (H-). Because H- is a strong nucleophile, the reaction follows an SN2 mechanism.
Answer to Problem 12.43P
The reagents needed to carry out the transformation is LiALH4+H2O to form the product
Explanation of Solution
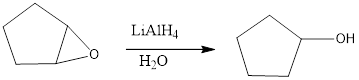
Because the reduction reaction follows an SN2 mechanism. The unhindered CH3X and 1 °
Reduction of the given transformation is carried out by using the LiALH4+H2O reagent.
(e)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is antihydroxylation product. Opening of the epoxy ring by OH anion and water to form the anti dihydroxylated product.
Answer to Problem 12.43P
The reagents needed to carry out the transformation is -OH+H2O to form the product
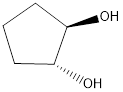
Explanation of Solution
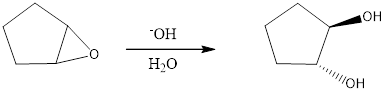
Epoxidation of cyclo-pentene adds an O atom from either above or below the plane of the double bond to form a single achiral epoxide. Opening of the epoxide ring occurs by the backside attack at either C - O bond. Because the epoxide is above the plane of the five-membered ring, nucleophilic attack occurs from below plane.
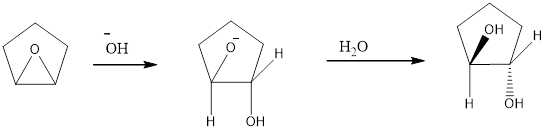
Reduction of the given transformation is carried out by using the -OH+H2O reagent.
(f)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is antihydroxylation product. Opening of the epoxy ring by OH anion and water to form the anti dihydroxylated product.
Answer to Problem 12.43P
The reagents needed to carry out the transformation is PBr3 or HBr to form the product
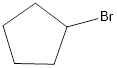
Explanation of Solution
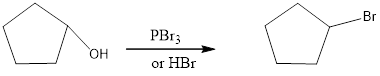
The bromo product is formed similar to SN2 fashion. Where bromo anion attack at the carbon where the alcohol attached so that bromo anion attack by the backside attack to for the bromo product.
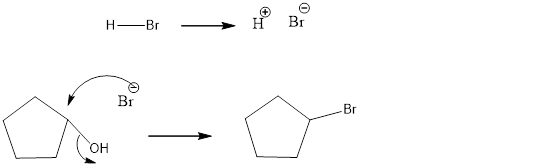
Reduction of the given transformation is carried out by using the PBr3 or HBr reagent.
(g)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
CrO3/H2SO4+H2O is an oxidizing agent. Oxidizing agents. CrO3 are strong, nonselective oxidants used in aqueous acid H2SO4+H2O. The general mechanism is given below.
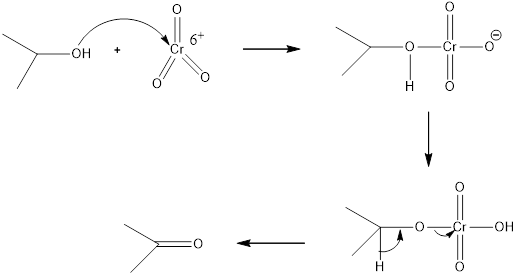
Answer to Problem 12.43P
The reagents needed to carry out the transformation is CrO3/H2SO4+H2O to form the product
Explanation of Solution
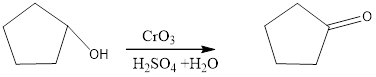
The oxidation of alcohols to carbonyl compounds is typically carried out by PCC or CrO3(where Cr6+ oxidants, which are reduced to Cr3+) to form the products. Oxidation of a 2 ° alcohol to an
Reduction of the given transformation is carried out by using the CrO3/H2SO4+H2O reagent.
(h)
Interpretation:
To identify the reagents needed to carry out the transformation
Concept introduction:
The given product is reduction product. Reduction of these C-X sigma bonds is an example of nucleophilic substitution, in which LiAlH4 serves as a source of a hydride nucleophile (H-). Because H- is a strong nucleophile, the reaction follows an SN2 mechanism
Answer to Problem 12.43P
The reagents needed to carry out the transformation is LiALH4+H2O to form the product

Explanation of Solution
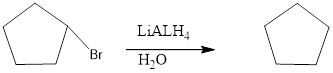
Because the reduction reaction follows an SN2 mechanism. The unhindered CH3X and 1 ° alkyl halides are more easily reduced than more substituted 2° and 3° halides. In unsymmetrical epoxides, nucleophilic attack of H- (from LiAIH4) occurs at the less substituted carbon atom.
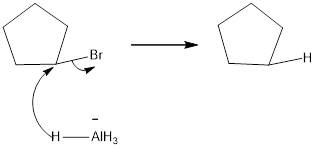
Reduction of the given transformation is carried out by using the LiALH4+H2O reagent.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
- Curved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.arrow_forwardWhat are the: нсе * Moles of Hice while given: a) 10.0 ml 2.7M ? 6) 10.ome 12M ?arrow_forwardYou are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.arrow_forward
- how to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forward
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
